Abstract
The accuracy of OptiMAL® dipsticks was compared with that of microscopy in the diagnosis of malaria infection in pregnancy. During the course of a clinical trial of antimalarial drugs in pregnancy, we screened 4500 pregnant women of all parities who accessed antenatal clinic services at St. Theresa’s Hospital’s in Nkoranza, Ghana, between March 2003 and December 2004 with OptiMAL® dipsticks and confirmed the diagnosis of malaria with microscopy. We determined the sensitivity, specificity, positive and negative predictive values, and the area under receiver operating characteristic (ROC) curve for the OptiMAL® antigen test compared to microscopy for the diagnosis of malaria infection in pregnancy. OptiMAL® dipsticks had a sensitivity of 96.6%, specificity of 85.4%, a positive predictive value of 92.7%, a negative predictive value of 92.6%, and an area under the ROC curve of 0.91 (95% CI of 0.90–0.92). The diagnostic accuracy of the OptiMAL® dipstick is high and the test may have practical use in the diagnosis of malaria infection in pregnancy in malaria endemic countries.
Keywords: malaria, diagnosis, pregnancy, OptiMAL dipstick, microscopy
Introduction
Early accurate diagnosis and prompt effective treatment of malaria are key components of the global malaria control strategy aimed at reducing unnecessary use of antimalarials but also preventing mortality and reducing morbidity (WHO 2005, 2006a). In settings where there are no facilities for laboratory diagnosis clinical diagnosis of malaria is recommended and made often based on the presence of fever or history of fever. Clinical diagnosis has never been validated and has very low accuracy as signs and symptoms of malaria may be non-specific. This results in increased morbidity and mortality, and increased transmission of drug-resistant parasite strains associated with inappropriate treatment (WHO 2006a). However, parasitological diagnosis ensures high specificity of malaria diagnosis, prevents unnecessary exposure to antimalarials, and helps to save cost on expensive antimalarials including artemisinin-based combination therapies (WHO 1999, 2003, 2005, 2006a; Njama-Meya et al 2007).
Methods used for parasitological diagnosis include light microscopy, fluorescent microscopy, rapid diagnostic tests (RDTs), and polymerase chain reaction (PCR). Light microscopy remains the method of choice for parasitological diagnosis of malaria worldwide. In comparison to PCR, it is highly sensitive and specific in expert hands at parasite densities above 50/μL (Moody 2002). Microscopy is beneficial for the identification of parasite species, quantification of parasite density, has lower cost, and is highly reproducible (WHO 1999, 2006a). Microscopy has other applications besides diagnosis of malaria, such as for the diagnosis of tuberculosis, schistosomiasis, and worm infestations. However, lack of expertise, limited supplies, inadequate maintenance of microscopes and reagents, and inadequate quality control make microscopy a less reliable technique in remote areas of malaria endemic regions.
Compared to microscopy, RDTs attract very low capital costs with little or no maintenance costs. They require only minimal training and are rapid, accurate, simple to conduct, and applicable in field and clinical settings in remote areas and in emergency situations where expert microscopy may not be practical (WHO 2006b). These major advantages of RDTs over microscopy notwithstanding, reports of assessments of their diagnostic performance have being inconsistent. Of particular concern is the variability in sensitivity and reliability of RDTs at both high and low levels of parasitemia and variable sensitivities in different geographic settings (Moody 2002; WHO 2006b). The excessive heat and humidity in tropical countries are likely to degrade the tests and reduce their shelf life. Prolonged exposure to temperatures greater than 30 °C is likely to reduce sensitivity, necessitating cold chains where possible for transport and storage (Moody 2002).
The PCR technique is used to detect sub-microscopic levels of parasitemia. It is a highly specialized technique requiring expensive equipment and very elaborate laboratory settings. It is not applicable to clinical settings but used mainly in research settings. It is often used as a standard against which the diagnostic accuracy of other parasitological methods is determined. Other methods such as fluorescent microscopy and laser desorption mass spectrometry may be used in malaria diagnosis but, like the PCR technique, they need special and expensive equipments and logistics and may not be applicable to most clinical settings (Moody 2002).
Malaria in pregnancy is a major public health problem with adverse consequences for the mother and her baby, particularly in sub-Saharan Africa (Menendez 1995; Menendez et al 2000; Shulman and Dorman 2003). However, the application of clinical and parasitological methods to diagnose malaria in pregnancy as recommended by the WHO (WHO 2004) has some challenges which limits the full deployment of effective treatment of malaria during pregnancy as a control measure. In highly endemic areas, malaria infection in pregnancy rarely causes an acute illness which makes clinical diagnosis of malaria during pregnancy difficult even though the infection may progress to cause severe anemia particularly in primigravidae. Microscopic diagnosis of malaria during pregnancy in women who live in endemic areas is limited by sequestration of parasitized red blood cells in the placental microcirculation, which reduces the number of circulating ring stage parasites detectable in peripheral blood by microscopy rendering the method insensitive in pregnancy (Mockenhaupt et al 2000) . The PCR technique and the laser desorption mass spectrometry (LDMS) may be useful for detecting parasitemia in pregnancy (Duffy and Fried 2005; Nyunt et al 2005). However, these techniques are not applicable in settings where the burden of malaria in pregnancy is greatest.
Malaria rapid diagnostic tests (RDTs) detect either histidine-rich protein-2 (HRP-2) or plasmodium lactate dehydrogenase enzyme (pLDH) (WHO 1999). Several studies have evaluated HRP-2 based RDTs (Kilian et al 1997; Tjitra et al 1999; Singh and Valecha 2000; Tarimo et al 2001; Forney et al 2003; Njama-Meya et al 2007) or pLDH based RDTs (Cooke et al 1999; Piper et al 1999; Iqbal et al 2003; Palmer et al 2003) for the diagnosis of malaria in children and non-pregnant adults. A few studies (Leke et al 1999; Mankhambo et al 2002; Mockenhaupt et al 2002; Singer et al 2004) have reported their use in the diagnosis of malaria at delivery and/or in the detection of placental malaria. The use of RDTs for diagnosing malaria in pregnant women attending antenatal clinics has been reported in only one study so far (VanderJagt et al 2005). We have, therefore, undertaken a study that compared the accuracy of the OptiMAL® antigen test for detecting peripheral parasitemia with microscopy in pregnant women at the time of presentation at an antenatal clinic during the course of a randomized controlled trial (RCT) of chloroquine (CQ) amodiaquine (AQ) and sulphadoxine-pyrimethamine (SP) for the treatment of malaria in pregnancy.
Methods
The study population comprised pregnant women of all parities who attended the antenatal clinic at the St. Theresa’s Hospital in, Nkoranza, Ghana between March 2003 and December 2004. All women who reported at the antenatal clinic and were presumed to be pregnant were invited to participate in the screening process. Prior to any clinical assessment and enrolment, pregnant women were assigned sequential screening numbers and screened for malaria infection using OptiMAL® dipsticks.
The OptiMAL® rapid malaria diagnosis test kits we used were bought in batches from the manufacturer, DiaMed AG—Cressier, Switzerland under license from Flow, Inc. Portland, Oregon, who also organized transportation to the study site on each occasion. All test kits were kept at room temperature at the study site and opened just before performing the test to protect against high humidity. Each test kit comprised a dipstick, pipette, conjugate and wash wells, and a dropper containing a buffer, sealed together in an aluminium package with a desiccant. The tests were performed and interpreted according to the manufacturer’s instructions. The four-member screening team comprised two senior secondary school and two polytechnic graduates who had received two weeks’ training from the principal investigator (HT) on the conduct and the interpretation of test results. Each member had a specific task assigned at each antenatal clinic screening session; one member performed the dipstick test, another prepared blood slides, one was in charge of record keeping, and the fourth member kept the time. The record keeper entered screening records on a screening form that had fields for the woman’s name, screening number, positive results, negative results, and fields to indicate whether enrolment took place. For each test, a new OptiMAL® kit was assembled. One drop of buffer solution was added to the conjugate well first, followed by adding 10 μL of finger-prick blood and the content was thoroughly mixed for about 1 minute. The dipstick was then placed in the conjugate well and allowed to stand for 10 minutes while the well content wick up the dipstick. The dipstick was then transferred to the wash well containing four drops of the buffer solution and allowed to wash for another 10 minutes. Then the reaction bands on the dipstick were read and interpreted.
Positive results were indicated by the presence of more than one red colored reaction bands on the dipstick. The top, middle and bottom reaction bands represented an internal control band, a pan-plasmodium-specific band and a Plasmodium falciparum-specific band respectively. The appearance of two test bands and one control band together indicated a P. falciparum infection; one middle test band and one control band indicated a P. vivax, P. malariae or P. ovale infection; one control band only at the top of the test strip was regarded as a negative test. A test with no reaction bands was considered invalid.
The screening team prepared thin and thick blood smears for each pregnant woman from a finger prick blood sample immediately after obtaining a sample for the OptiMAL® test. Blood slides of women who had a positive OptiMAL® test together with a set of randomly selected slides of women who had a negative OptiMAL® test were examined by a microscopist blinded to the OptiMAL® test result. The negative OptiMAL® test slides were selected using the serial screening numbers of negative OptiMAL® test results. Every fifth number was selected and the corresponding slide examined along with the positive test slides obtained during each screening session. A blood slide was deemed to be negative only when examination of 100 high power fields of a thick film did not show the presence of sexual or asexual forms of P. falciparum. A randomly selected sample of 10% of both negative and positive slides was re-examined by a microscopist (CA) at the Noguchi Memorial Institute of Medical Research for quality assurance.
Stata (version 8.1) software was used to clean and validate data and to calculate the test validation features according to defined standards (Altman and Bland 1994a, b, c; Deeks and Altman 2004). For the slides that were re-examined by a second microscopist, an interobserver variability in the interpretation of microscopy results was computed using the kappa statistic including 95% confidence intervals (CI).
The clinical trial on which this study was based was approved by the ethics committee of the London School of Hygiene and Tropical Medicine and by the Health Research Unit of the Ministry of Health of Ghana It was registered on the NIH Clinical trials register and assigned the number NCT00131703.
Results
The overall prevalence of peripheral parasitemia among antenatal clinic attenders was 22%, with monthly variations ranging from 9% to 32% in 2003 and from 16% to 34% in 2004. The prevalence of parasitemia peaked in June, July and August in 2003 (the rainy season) but no seasonality was observed in 2004. The main parasite species detected was P. falciparum; no mixed infections were detected in the study population either by OptiMAL® test or microscopy.
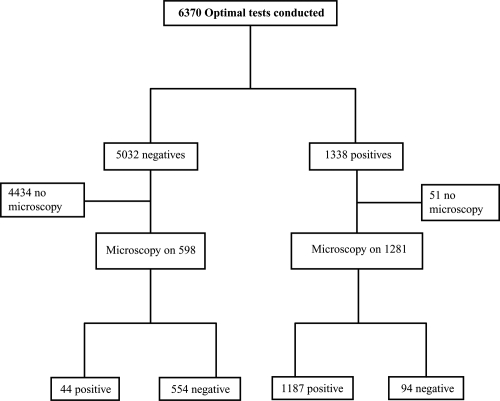
A total of 6370 OptiMAL® tests were performed on 4500 pregnant women, 1338 (21%) tests were positive and 5032 were negative (Figure 1). Ninety-four slides of 1281 OptiMAL® positive women assessed microscopically were negative while 44 (7.3%) of 598 slides obtained from OptiMAL® test negative women were positive on microscopy. Fifty-seven women who had a positive OptiMAL® test did not have a blood slide collected because they declined consent before further samples could be taken or the screening process completed.
Figure 1.
Study profile.
Inter-observer variability in reading blood slides by microscopists from the St. Theresa’s Hospital and Noguchi Memorial Institute of Medical Research was minimal (kappa 0.34; 95% CI 0.19–0.49).
Low parity, gravidity, and age were associated significantly with positive dipstick tests (p < 0.001) (data not shown). Nulliparous women (RR = 3.3; 95% CI, 3.0–3.7) and primiparous women (RR = 1.8; 95% CI, 1.5–2.0) respectively were more likely to have positive dipstick tests compared to multiparous women. Similarly primigravidae (RR = 3.2; 95% CI, 2.9–3.6) and secundigravidae (RR = 1.9; 95% CI, 1.7–2.2) were more likely than multigravidae to have positive dipstick results. Pregnant women less than 30 years of age were more likely than those 30 years or above to have positive dipstick results (RR = 2.4; 95% CI, 2.1–2.6). Gestational age at screening appears to be associated with a positive dipstick test result (p = 0.01); but there was no difference testing positive between women screened in their second trimester and those screened in their third trimester (RR = 1.1; 95% CI, 1.0–1.2).
The diagnostic accuracy of the OptiMAL® dipstick increased with increasing density of parasitemia (Tables 1 and 2). The sensitivity of the test was 57.1%, with a specificity of 93.3% if parasitemia was below 50/μL. However, for parasite densities above 50/ μL, the sensitivity and specificity of the test were 100% and 93.3%, respectively. The predictive values increased with higher parasite density. The area under a non-parametric receiver operating characteristic (ROC) curve comparing the performance of OptiMAL dipsticks with microscopy in the diagnosis of malaria in pregnancy was 0.91 (0.9–0.92).
Table 1.
The performance of the OptiMAL® test versus peripheral microscopy in the diagnosis of malaria infection in pregnancy
| Microscopy |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasite level <50/μL |
Parasite level 50–100/μL |
Parasite level >100/μL |
Overall assessment |
|||||||||
| Positive | Negative | Total | Positive | Negative | Total | Positive | Negative | Total | Positive | Negative | Total | |
| OptiMAL® test | ||||||||||||
| Positive | 24 | 9 | 33 | 68 | 9 | 77 | 822 | 9 | 831 | 1187 | 94 | 1281 |
| Negative | 18 | 125 | 143 | 0 | 125 | 125 | 0 | 125 | 125 | 44 | 554 | 598 |
| Total | 42 | 134 | 176 | 68 | 134 | 202 | 822 | 134 | 956 | 1231 | 648 | 1879 |
Table 2.
Performance of the OptiMAL® dipstick according to the level of peripheral parasitemia during pregnancy
| Parasitemia (parasites/μL of whole blood) | Sensitivity (% [95% CI]) | Specificity (% [95% CI]) | Positive PV (% [95% CI]) | Negative PV (% [95% CI]) | Accuracy | Likelihood ratio |
Area under ROC curve (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Positive test | Negative test | |||||||
| <50 | 57.1(49.8–64.5) | 93.3 (89.6–97) | 72.7 (66.6–79.3) | 87.4 (82.5–92.3) | 84.7 | 8.5 | 0.46 | 0.75 (0.67–0.83) |
| 50–100 | 100 | 93.3 (89.8–96.7) | 88.3 (83.9–92.7) | 100 | 95.5 | 14.9 | <0.001 | 0.97 (0.95–0.99) |
| >100 | 100 | 93.3 (91.7–94.9) | 98.9 (98.3–99.6) | 100 | 99.1 | 14.9 | <0.001 | 0.97 (0.95–0.99) |
| Overall Assessment (Positive/Negative) | 96.4 (95.6–97.3) | 85.5 (83.9–87.1) | 92.7 (91.5–93.8) | 92.6 (91.5–93.8) | 92.7 | 6.6 | 0.04 | 0.91 (0.9–0.92) |
Abbreviations: PV, predictive value; ROC, receiver operating characteristics.
Discussion
This study has shown that the OptiMAL® dipstick was able to identify more than 90% of pregnant women with peripheral blood malaria parasitmia and correctly labeled about 85% of non-parasitemic pregnant women. However, the test performed poorly if the parasite density was below 50/μL.
A limitation of this study was the use of peripheral blood microscopy as the “gold standard” for the diagnosis of malaria infection. The accuracy of a diagnosis based on microscopy depends heavily on the expertise of the microscopist. However, the study microscopists were very experienced and there was good agreement between the first and second microscopist. A more serious constraint is that it is known that there may be malaria infection of the placenta in the absence of peripheral blood parasitemia (Mockenhaupt et al 2000, 2002) and, in contrast to studies which have investigated women at the time of delivery, we were unable to determine whether the placenta was infected. It is possible that in the case of some women with a positive OptiMAL® test but a negative blood film, the RDT was detecting a placental malaria infection.
Studies of the use of rapid diagnostic tests in pregnant women have been undertaken mainly for the purpose of diagnosing placental malaria at delivery (Leke et al 1999; Mankhambo et al 2002; Singer et al 2004). All of these studies reported the tests as having good diagnostic features apart from one (Mankhambo et al 2002), which casts doubts on the sensitivity of the OptiMAL® test for diagnosing placental malaria. A recently published study which reported the use of OptiMAL® dipsticks in the diagnosis of malaria in pregnant women who were attending antenatal clinics in Nigeria (VanderJagt et al 2005) found the test to be insensitive compared to microscopy and PCR. However, it is not possible to compare the results of this study to ours because the authors did not report the sensitivity, specificity, predictive values, or likelihood ratios, the indicators required for comparing the performance of diagnostic tests.
The OptiMAL® dipstick test is expected to produce similar results in all environments if the test is conducted according to the manufacturer’s instructions. However, we found that during the dry months of the year, the wash well dried up too soon if the manufacturer’s instruction of filling this well at the beginning of the test process was followed. Under these conditions, optimum results were obtained when the wash well was filled just before the dipstick was transferred into it.
Screening with the OptiMAL® dipsticks fitted well into the routine of the antenatal clinic schedule and use of the RDT caused minimal disruptions to the flow of work at the clinic throughout the study period. In the past, the high cost of RDT kits (US$1.00 to US$3.50 per test) compared to the cost of US$0.40 for microscopy (Hanson et al 2004) coupled with the availability of relatively cheap and sensitive drugs (chloroquine and sulphadoxine-pyrimethamine [SP]) for treatment of malaria has been used to argue that RDT use in moderate to high transmission areas may not be cost effective (Wongsrichanalai 2001; Fernando et al 2004; Hanson et al 2004). However, in Ghana the average costs of microscopic diagnosis of malaria charged by public and mission hospitals in the Brong Ahafo region during the study period were 10,000 and 15,000 cedis (approximately US$1.1 and US$1.6, respectively) more than the US$1.00 cost per test of the OptiMAL® dipstick used in the present study. Thus, cost may no longer be an issue limiting the wider use of RDTs in routine health care in Ghana. However the fact that RDTs are not quantitative is a limiting factor (Wongsrichanalai 2001). Furthermore, it is not clear to what extent antenatal rapid antigen tests results can predict placental parasitemia. Nevertheless, RDTs appear more sensitive in detecting sub microscopic parasitemia in pregnancy and probably placental parasitemia compared to traditional microscopy (Mockenhaupt et al 2002).
Since resistance to SP is increasing and an effective and safe antimalarial drug to replace SP for intermittent preventive treatment is yet to be identified it is worthwhile considering the option of detecting malaria infection using rapid diagnostic tests and treating only those who are infected with an effective antimalarial drug combination as opposed to giving an antimalarial to all pregnant women attending an antenatal clinic. In this context the use of OptiMAL® dipsticks to detect circulating pLDH may be of public health significance. Antenatal RDT screening and treatment of only those with positive results would: (1) reduce unnecessary antimalarial drug use, thus offsetting the cost of using rapid diagnostic tests (Hanson et al 2004); (2) reduce drug pressure, which is implicated in the spread of parasite resistance to antimalarial drugs (Wernsdorfer 1994; Wongsrichanalai 2001); (3) limit exposure of pregnant women to the newer and more expensive antimalarial drugs only to those who are infected. It is worthwhile examining the effects of restricting effective malaria treatment to those who are found to be infected with malaria during antenatal.
References
- Altman DG, Bland JM. Statistics notes: Diagnostic tests 1: Sensitivity and specificity. BMJ. 1994a;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Statistics notes: Diagnostic tests 3: Receiver operating characteristic plots. BMJ. 1994b;309:188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Statistics notes: Diagnostic tests 2: Predictive values. BMJ. 1994c;309:102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke AH, Chiodini PL, Doherty T, et al. Comparison of a parasite lactate dehydrogenase-based immunochromatographic antigen detection assay (optimal (r)) with microscopy for the detection of malaria parasites in human blood samples. Am J Trop Med Hyg. 1999;60:173–6. doi: 10.4269/ajtmh.1999.60.173. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG. Diagnostic tests 4: Likelihood ratios. BMJ. 2004;329:168–9. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P, Fried M. Malaria: new diagnostics for an old problem. Am J Trop Med Hyg. 2005;73:482–3. [PubMed] [Google Scholar]
- Fernando SD, Karunaweera ND, Fernando WP, et al. A cost analysis of the use of the rapid, whole-blood, immunochromatographic p.F/p.V assay for the diagnosis of plasmodium vivax malaria in a rural area of sri lanka. Ann Trop Med Parasitol. 2004;98:5–13. doi: 10.1179/000349804225003064. [DOI] [PubMed] [Google Scholar]
- Forney JR, Wongsrichanalai C, Magill AJ, et al. Devices for rapid diagnosis of malaria:Evaluation of prototype assays that detect plasmodium falciparum histidine-rich protein 2 and a plasmodium vivax-specific antigen. J Clin Microbiol. 2003;41:2358–66. doi: 10.1128/JCM.41.6.2358-2366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K, Goodman C, Lines J, et al. Global Forum for Health Research. WHO; 2004. The economics of malaria control interventions. [Google Scholar]
- Iqbal J, Muneer A, Khalid N, et al. Performance of the optimal test for malaria diagnosis among suspected malaria patients at the rural health centers. Am J Trop Med Hyg. 2003;68:624–8. doi: 10.4269/ajtmh.2003.68.624. [DOI] [PubMed] [Google Scholar]
- Kilian AH, Mughusu EB, Kabagambe G, et al. Comparison of two rapid, hrp2-based diagnostic tests for plasmodium falciparum. Trans R Soc Trop Med Hyg. 1997;91:666–7. doi: 10.1016/s0035-9203(97)90514-9. [DOI] [PubMed] [Google Scholar]
- Leke RF, Djokam RR, Mbu R, et al. Detection of the plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women:Implications for diagnosing placental malaria. J Clin Microbiol. 1999;37:2992–6. doi: 10.1128/jcm.37.9.2992-2996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankhambo L, Kanjala M, Rudman S, et al. Evaluation of the Optimal® rapid antigen test and species-specific pcr to detect placental plasmodium falciparum infection at delivery. J Clin Microbiol. 2002;40:155–8. doi: 10.1128/JCM.40.1.155-158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–83. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–5. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt FP, Rong B, Till H, et al. Submicroscopic plasmodium falciparum infections in pregnancy in ghana. Trop Med Int Health. 2000;5:167–73. doi: 10.1046/j.1365-3156.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt FP, Ulmen U, von Gaertner C, et al. Diagnosis of placental malaria. J Clin Microbiol. 2002;40:306–8. doi: 10.1128/JCM.40.1.306-308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njama-Meya D, Clark TD, Nzarubara B, et al. Treatment of malaria restricted to laboratory-confirmed cases: a prospective cohort study in Ugandan children. Malar J. 2007;6:7. doi: 10.1186/1475-2875-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunt M, Pisciotta J, Feldman AB, et al. Detection of plasmodium falciparum in pregnancy by laser desorption mass spectrometry. Am J Trop Med Hyg. 2005;73:485–90. [PubMed] [Google Scholar]
- Palmer CJ, Bonilla JA, Bruckner DA, et al. Multicenter study to evaluate the optimal test for rapid diagnosis of malaria in U.S. hospitals. J Clin Microbiol. 2003;41:5178–82. doi: 10.1128/JCM.41.11.5178-5182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper R, Lebras J, Wentworth L, et al. Immunocapture diagnostic assays for malaria using plasmodium lactate dehydrogenase (pldh) Am J Trop Med Hyg. 1999;60:109–18. doi: 10.4269/ajtmh.1999.60.109. [DOI] [PubMed] [Google Scholar]
- Shulman CE, Dorman EK. Reducing childhood mortality in poor countries - importance and prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg. 2003;97:30–5. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- Singer LM, Newman RD, Diarra A, et al. Evaluation of a malaria rapid diagnostic test for assessing the burden of malaria during pregnancy. Am J Trop Med Hyg. 2004;70:481–5. [PubMed] [Google Scholar]
- Singh N, Valecha N. Evaluation of a rapid diagnostic test, ‘determine malaria pf’, in epidemic-prone, forest villages of central india (madhya pradesh) Ann Trop Med Parasitol. 2000;94:421–7. doi: 10.1080/00034983.2000.11813560. [DOI] [PubMed] [Google Scholar]
- Tarimo DS, Minjas JN, Bygbjerg IC. Malaria diagnosis and treatment under the strategy of the integrated management of childhood illness (imci):Relevance of laboratory support from the rapid immunochromatographic tests of ict malaria p.F/p.V and optimal. Ann Trop Med Parasitol. 2001;95:437–44. doi: 10.1080/13648590120068971. [DOI] [PubMed] [Google Scholar]
- Tjitra E, Suprianto S, Dyer M, et al. Field evaluation of the ict malaria p.F/p.V immunochromatographic test for detection of plasmodium falciparum and plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern indonesia. J Clin Microbiol. 1999;37:2412–17. doi: 10.1128/jcm.37.8.2412-2417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderJagt TA, Ikeh EI, Ujah IO, et al. Comparison of the Optimal® rapid test and microscopy for detection of malaria in pregnant women in Nigeria. Trop Med Int Health. 2005;10:39–41. doi: 10.1111/j.1365-3156.2004.01349.x. [DOI] [PubMed] [Google Scholar]
- Wernsdorfer WH. Epidemiology of drug resistant malaria. Acta Tropica. 1994;56:143–56. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- WHO. New Perspectives: Malaria Diagnosis - Report of a Joint WHO/USAID Informal Consultation. Geneva: 1999. WHO/CDS/RBM/2000.14. [Google Scholar]
- WHO. Malaria control in the WHO African Region – Turning resources into results: Annual Report. 2003 [Google Scholar]
- WHO. A strategic framework for malaria prevention and control during pregnancy in the african region. Brazzaville: WHO Regional Office for Africa; 2004. AFR/MAL/04/01. [Google Scholar]
- WHO. World Malaria Report – 2005. Geneva: World Health Organization and UNICEF; 2005. [Google Scholar]
- WHO. WHO guidelines for the treatment of malaria. 2006a WHO/HTM/MAL/2006.1108. [Google Scholar]
- WHO. The role of laboratory diagnosis to support malaria disease management. Focus on the use of rapid diagnostic tests in areas of high transmission – a report of a WHO technical consultation. 2006b [Google Scholar]
- Wongsrichanalai C. Rapid diagnostic techniques for malaria control. Trends in Parasitology. 2001;17:307–309. doi: 10.1016/s1471-4922(01)01925-0. [DOI] [PubMed] [Google Scholar]