Abstract
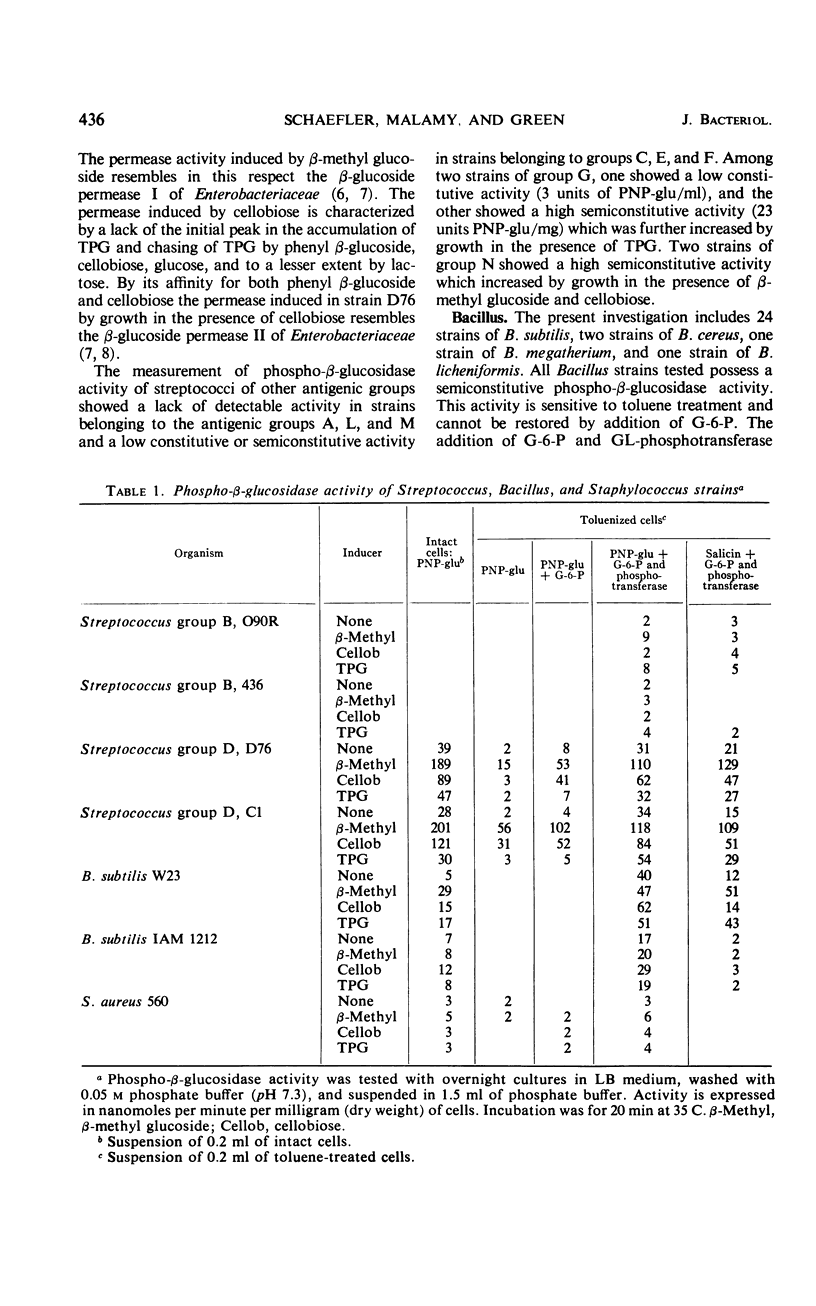
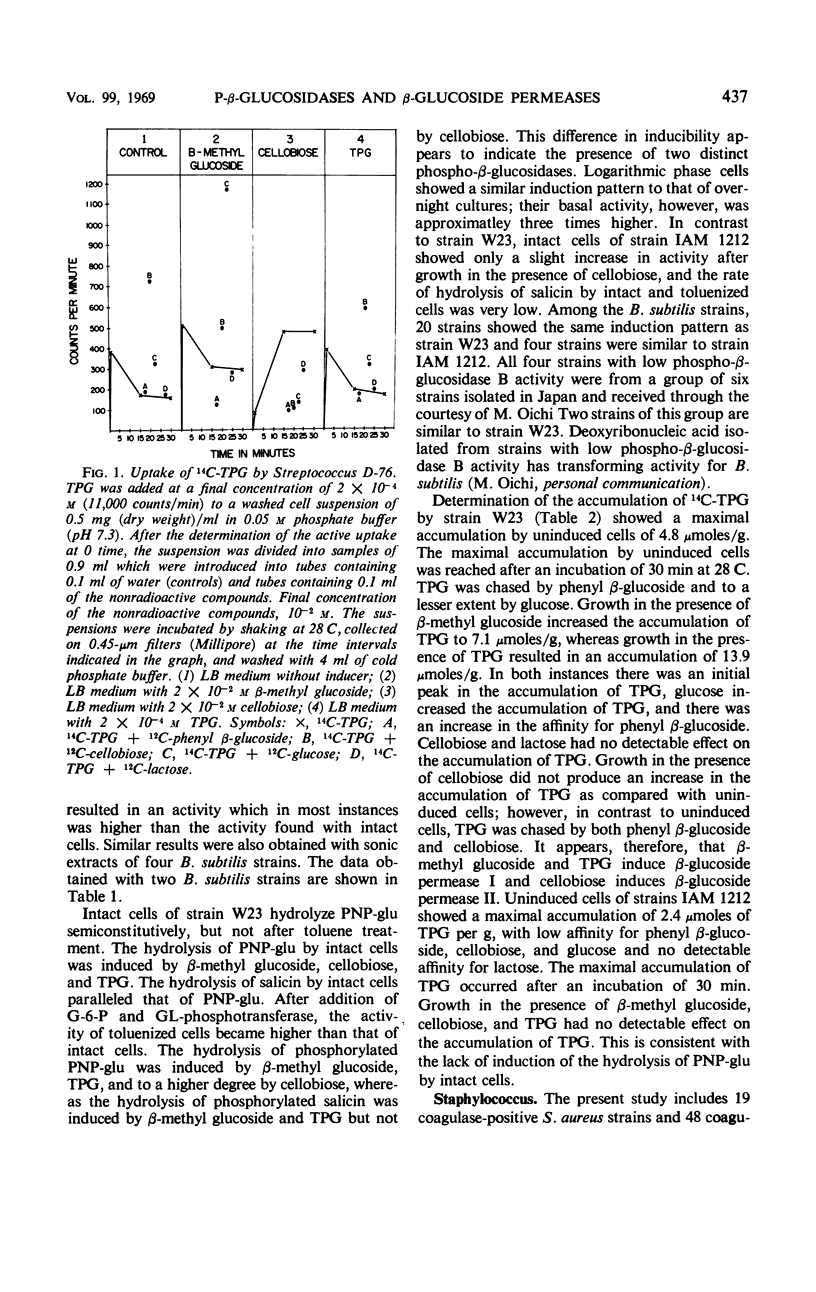
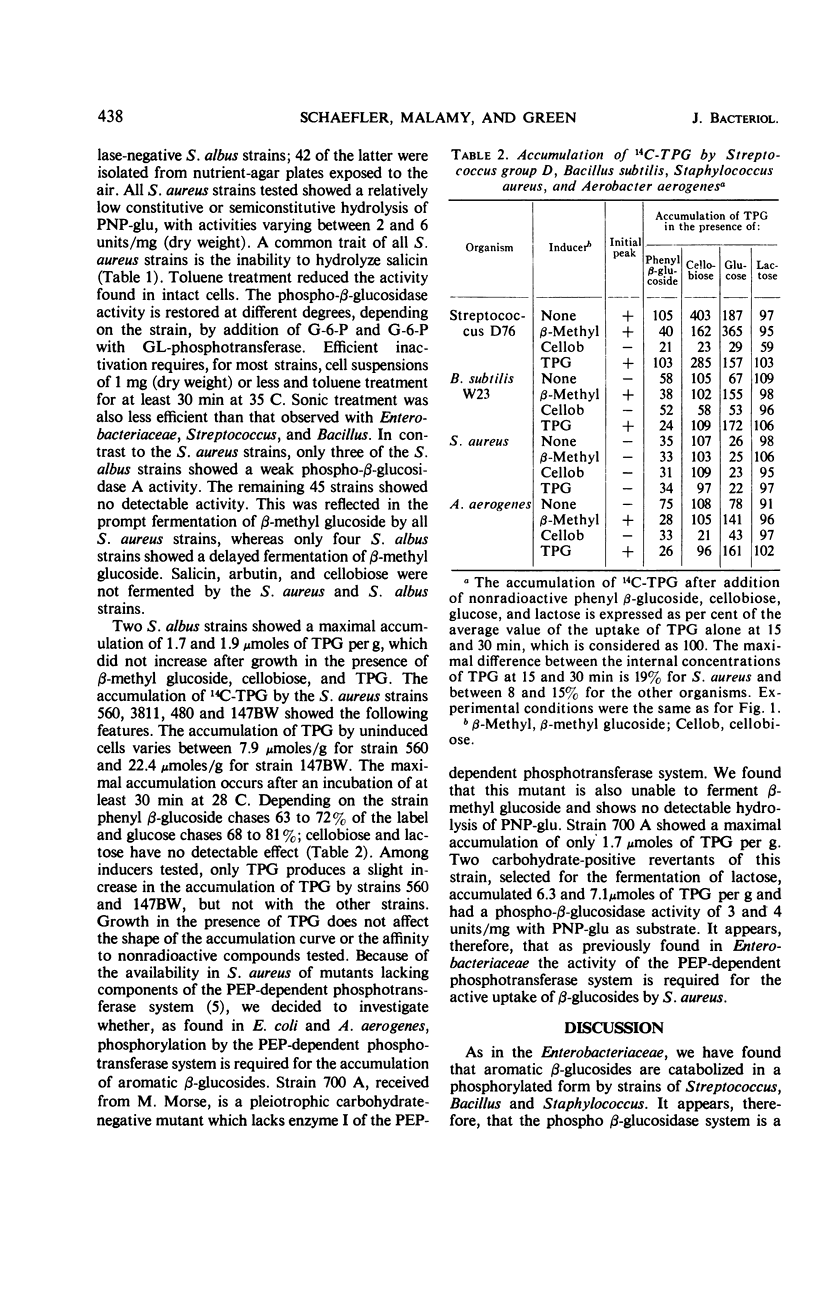
The presence of phospho-β-glucosidases and β-glucoside permeases was found in strains of Streptococcus, Bacillus, and Staphylococcus. In streptococci, the phospho-β-glucosidase activity depends on the antigenic group. The highest activity was found in strains of group D. In group D strains, phospho-β-glucosidase activity is induced by β-methyl glucoside and cellobiose but not by thiophenyl β-glucoside (TPG). With the exception of four strains isolated in Japan, all strains of B. subtilis tested possess an inducible phospho-β-glucosidase activity, β-methyl glucoside, cellobiose, and TPG acting as inducers. S. aureus strains possess phospho-β-glucosidase A but not phospho-β-glucosidase B, whereas most S. albus strains show no detectable phospho-β-glucosidase activity. The prompt fermentation of β-methyl glucoside by S. aureus strains could serve as an additional criterion for their differentiation from S. albus. A comparative investigation of the active uptake of 14C-TPG showed that a Streptococcus group D strain and a B. subtilis strain posses two inducible permeases with characteristics similar to the β-glucoside permeases I and II of Enterobacteriaceae. In S. aureus, TPG is accumulated by a constitutive permease with high affinity for aromatic β-glucosides and glucose. The active uptake of TPG by S. aureus appears to depend on the activity of the phosphoenol pyruvate-dependent phosphotransferase system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. K. Purification and specificity of cellobiose phosphorylase from Clostridium thermocellum. J Biol Chem. 1968 Jun 10;243(11):2899–2904. [PubMed] [Google Scholar]
- HENRIKSON C. V., SMITH P. F. BETA-GLUCOSIDASE ACTIVITY IN MYCOPLASMA. J Gen Microbiol. 1964 Oct;37:73–80. doi: 10.1099/00221287-37-1-73. [DOI] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Malamy A. Taxonomic investigations on expressed and cryptic phospho-beta-glucosidases in Enterobacteriaceae. J Bacteriol. 1969 Aug;99(2):422–433. doi: 10.1128/jb.99.2.422-433.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Schenkein I. Beta-glucoside permeases and phospho beta-glucosidases in Aerobacter aerogenes: relationship with cryptic phospho beta-glucosidases in Enterobacteriaceae. Proc Natl Acad Sci U S A. 1968 Jan;59(1):285–292. doi: 10.1073/pnas.59.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]



