Abstract
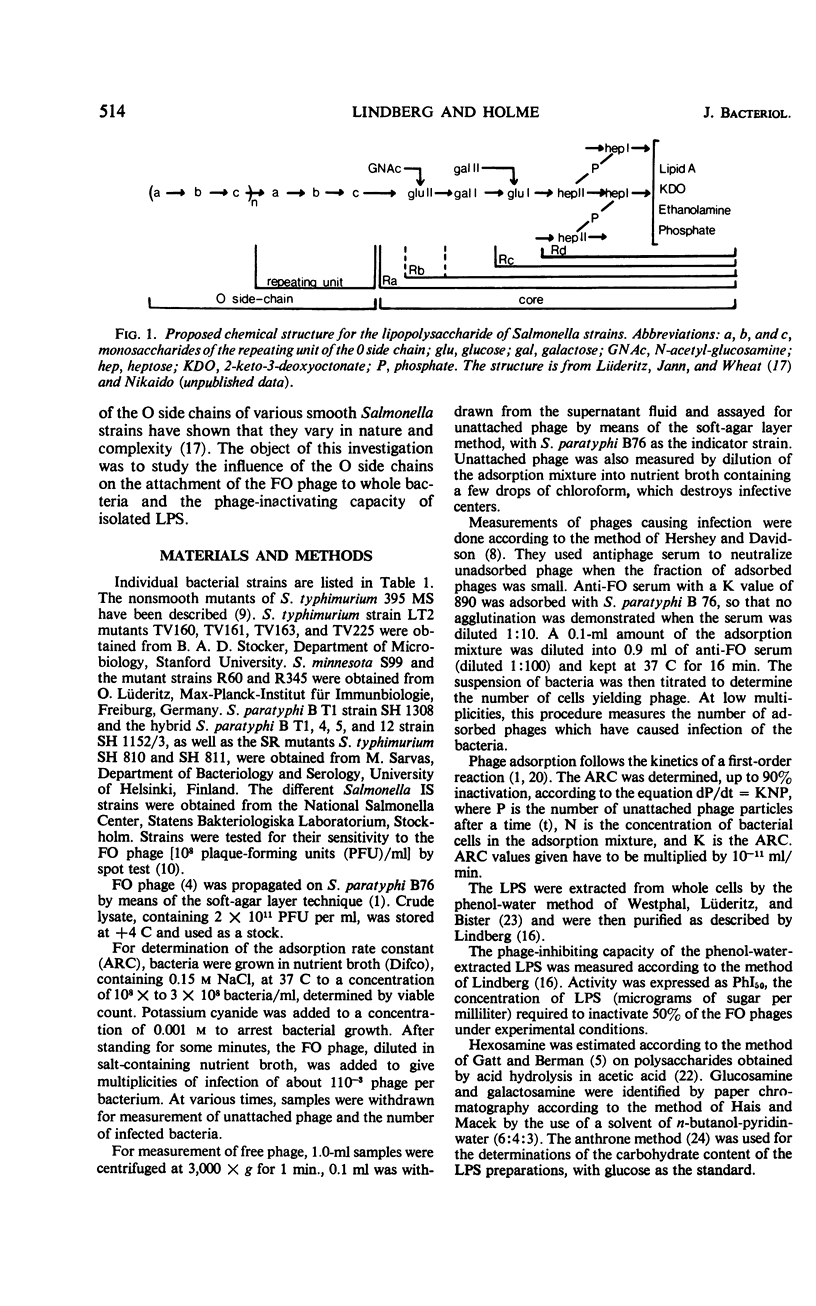
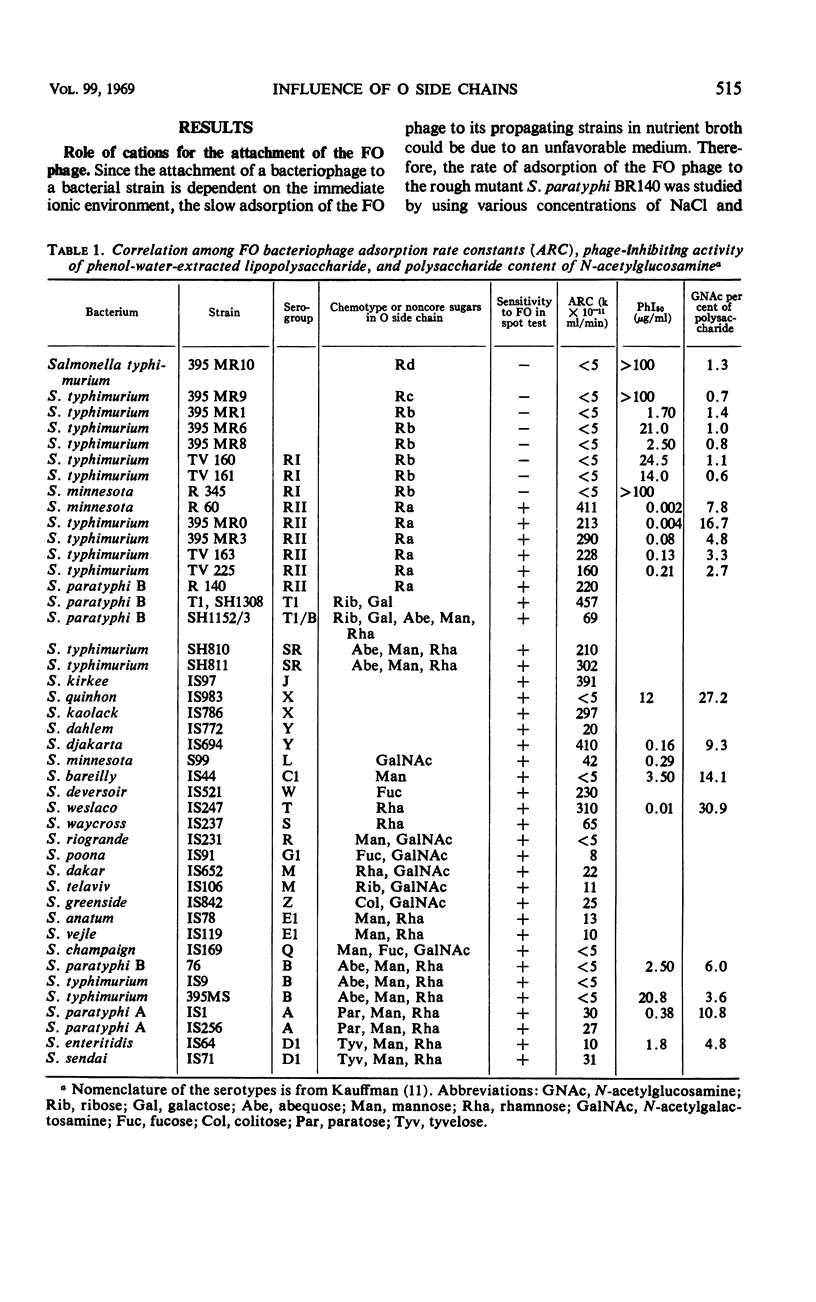
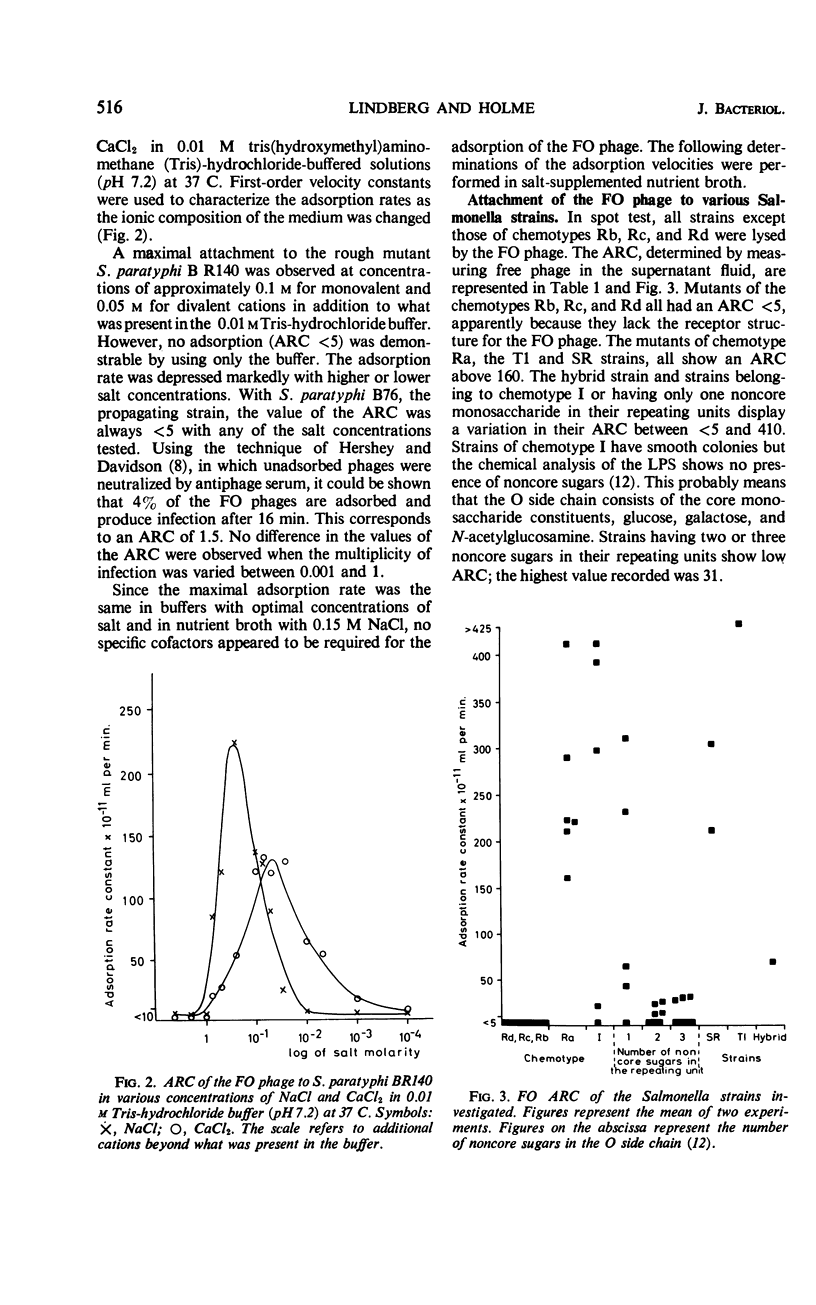
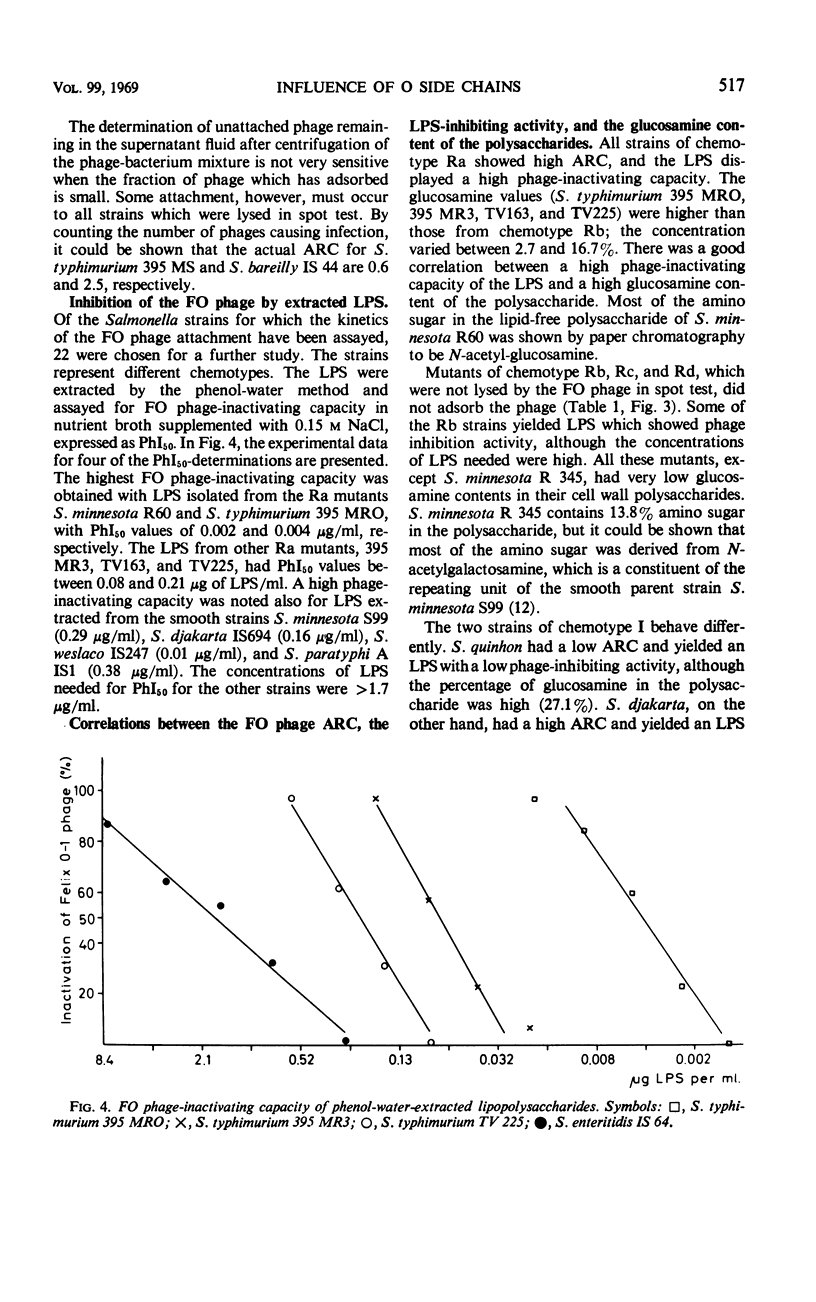
The adsorption rate constant (ARC) of the Felix O-1 (FO) bacteriophage to sensitive Salmonella strains was used to determine the effect of variations in surface antigens on phage attachment. The N-acetylglucosamine of the common-core polysaccharide of the Salmonella lipopolysaccharide (LPS) was found to be an essential part of the receptor for the FO phage in conformation with earlier reports. It was found that (i) the ARC was low for strains having O side chains containing two or three non core monosaccharides, (ii) the ARC varied when the O side chain contained no, or only one, noncore monosaccharide, (iii) the ARC was high when the O side chain contained only one repeating unit, and (iv) the ARC was high to mutants of chemotype Ra in which the N-acetylglucosamine was the terminal sugar of the LPS. Since a good correlation was found between the ARC of the FO phage and the phage-inactivating capacity of phenol water-extracted LPS, the results suggest that only the structure and composition of the LPS determines the adsorption rate of the FO phage. The phage-inactivating capacity of LPS from the Ra mutants increased in parallel with higher glucosamine contents in the core polysaccharide. In smooth strains having long and numerous O side chains, the access of the FO phage to its receptor is probably blocked by the presence of the side chains, whereas short and numerous side chains or T1 side chains do not interfere with the FO attachment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gatt R., Berman E. R. A rapid procedure for the estimation of amino sugars on a micro scale. Anal Biochem. 1966 Apr;15(1):167–171. doi: 10.1016/0003-2697(66)90262-4. [DOI] [PubMed] [Google Scholar]
- Hershey A D, Davidson H. Allelic and Nonallelic Genes Controlling Host Specificity in a Bacteriophage. Genetics. 1951 Nov;36(6):667–675. doi: 10.1093/genetics/36.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFFMANN F., LUEDERITZ O., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. I. Analysis of the sugar component of Salmonella O antigens]. Zentralbl Bakteriol. 1960 May;178:442–458. [PubMed] [Google Scholar]
- Kallings L. O. Sensitivity of various salmonella strains to felix 0-1 phage. Acta Pathol Microbiol Scand. 1967;70(3):446–454. doi: 10.1111/j.1699-0463.1967.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Studies of a receptor for felix O-1 phage in Salmonella minnesota. J Gen Microbiol. 1967 Aug;48(2):225–233. doi: 10.1099/00221287-48-2-225. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Naide Y., Mäkelä P. H. Biosynthesis of O-antigenic polysaccharides in Salmonella. Ann N Y Acad Sci. 1966 Jun 30;133(2):299–314. doi: 10.1111/j.1749-6632.1966.tb52373.x. [DOI] [PubMed] [Google Scholar]
- Sarvas M., Lüderitz O., Westphal O. Immunochemical studies on T1,S hybrids of Salmonella paratyphi--B. Ann Med Exp Biol Fenn. 1967;45(2):117–126. [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Lüderitz O., Westphal O. Studies on the structure of lipopolysaccharides of Salmonella minnesota and Salmonella typhimurium R strains. Biochem J. 1965 Aug;96(2):439–448. doi: 10.1042/bj0960439. [DOI] [PMC free article] [PubMed] [Google Scholar]


