Abstract
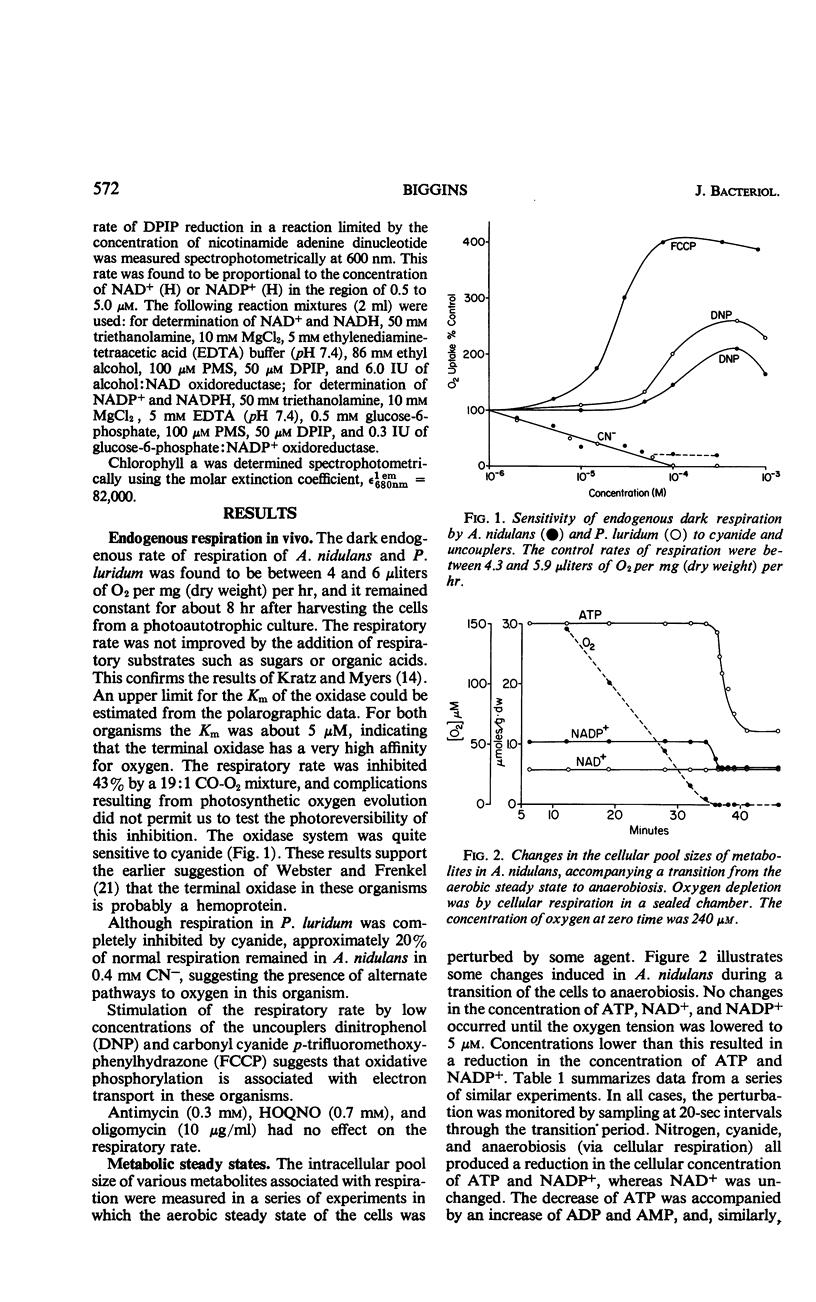
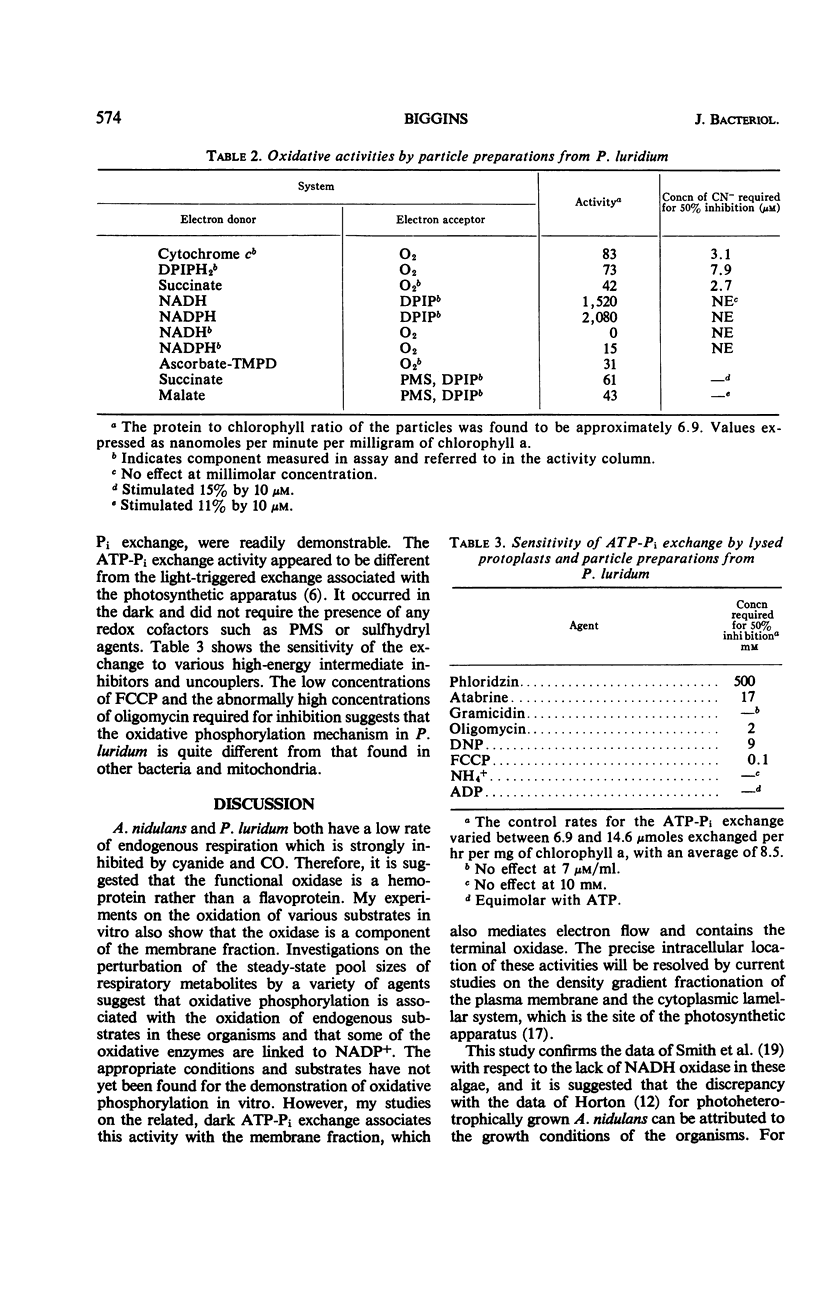
The low rate of endogenous respiration exhibited by the blue-green algae Anacystis nidulans and Phormidium luridum was not increased by the addition of respiratory substrates. However, endogenous respiration was inhibited by low concentrations of cyanide and by high carbon monoxide tensions. In addition, the uncouplers dinitrophenol and carbonyl cyanide p-trifluoromethoxyphenylhydrazone both stimulated the respiratory rate. The transition of cells from the aerobic steady state to anaerobiosis was accompanied by a decrease in the concentration of cellular nicotinamide adenine dinucleotide phosphate (NADP+) and adenosine triphosphate (ATP), whereas the concentration of nicotinamide adenine dinucleotide (NAD+) was unchanged. Concomitant with the metabolite decreases were stoichiometric increases io reduced NADP+ (NADPH), adenosine diphosphate, and adenosine monophosphate. A decrease in ATP was also observed after the addition of uncouplers. These data are interpreted as evidence for the association of oxidative phosphorylation with the oxidation of NADP+-linked substrates in these algae. Membrane fragments isolated from the algal cells oxidized succinate, malate, ferrocytochrome c, ascorbate-tetramethyl-p-phenylenediamine, and reduced 2,6-dichlorophenol indophenol but did not oxidize NADPH or reduced NAD+ in a cyanide-sensitive system. Oxidative phosphorylation has not yet been demonstrated in these fragments, but a dark ATP-Pi exchange, distinct from the lighttriggered exchange associated with photosynthesis, is readily observed. This exchange was inhibited by phloridzin, Atabrine, and uncouplers in concentrations which suggest that the mechanism of oxidative phosphorylation in blue-green algae is different from that found in other bacteria and in mitochondria. These results led to the conclusion that the biochemical basis for obligate autotrophy in these organisms does not lie in the metabolic events associated with terminal electron transport and energy conservation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Biggins J., Dietrich W. E., Jr Respiratory mechanisms in the Flexibacteriaceae. I. Studies on the terminal oxidase system of Leucothrix mucor. Arch Biochem Biophys. 1968 Oct;128(1):40–50. doi: 10.1016/0003-9861(68)90007-6. [DOI] [PubMed] [Google Scholar]
- Biggins J. Photosynthetic Reactions by Lysed Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1447–1456. doi: 10.1104/pp.42.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J. Preparation of Metabolically Active Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1442–1446. doi: 10.1104/pp.42.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli C., Avron M. A light-triggered adenosine triphosphate-phosphate exchange reaction in chloroplasts. Eur J Biochem. 1967 Oct;2(3):318–326. doi: 10.1111/j.1432-1033.1967.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Gibbs M. Dark and photometabolism of sugars by a blue green alga: Tolypothrix tenuis. Plant Physiol. 1966 Apr;41(4):731–737. doi: 10.1104/pp.41.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane W. C., Sr, Hohl M. C., Krogmann D. W. Photophosphorylation activity in cell-free preparations of a blue-green alga. Biochim Biophys Acta. 1965 Sep 27;109(1):108–116. doi: 10.1016/0926-6585(65)90095-6. [DOI] [PubMed] [Google Scholar]
- ECHLIN P., MORRIS I. THE RELATIONSHIP BETWEEN BLUE-GREEN ALGAE AND BACTERIA. Biol Rev Camb Philos Soc. 1965 May;40:143–187. doi: 10.1111/j.1469-185x.1965.tb00800.x. [DOI] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Holm-Hansen O. Ecology, physiology, and biochemistry of blue-green algae. Annu Rev Microbiol. 1968;22:47–70. doi: 10.1146/annurev.mi.22.100168.000403. [DOI] [PubMed] [Google Scholar]
- Horton A. A. NADH oxidase in blue-green algae. Biochem Biophys Res Commun. 1968 Sep 6;32(5):839–845. doi: 10.1016/0006-291x(68)90317-3. [DOI] [PubMed] [Google Scholar]
- JONES L. W., MYERS J. A COMMON LINK BETWEEN PHOTOSYNTHESIS AND RESPIRATION IN A BLUE-GREEN ALGA. Nature. 1963 Aug 17;199:670–672. doi: 10.1038/199670a0. [DOI] [PubMed] [Google Scholar]
- Lang N. J. The fine structure of blue-green algae. Annu Rev Microbiol. 1968;22:15–46. doi: 10.1146/annurev.mi.22.100168.000311. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. A chlorophyll-containing cell fraction from the blue-green alga, Anabaena variabilis. J Biophys Biochem Cytol. 1960 Jun;7:583–584. doi: 10.1083/jcb.7.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., VAN NIEL C. B. The concept of a bacterium. Arch Mikrobiol. 1962;42:17–35. doi: 10.1007/BF00425185. [DOI] [PubMed] [Google Scholar]
- Sauer K., Biggins J. Action spectra and quantum yields for nicotinamide--adenine dinucleotide phosphate reduction by chloroplasts. Biochim Biophys Acta. 1965 May 25;102(1):55–72. doi: 10.1016/0926-6585(65)90202-5. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B., Sträuli U. An assay procedure for nicotinamide-adenine dinucleotides in rat liver and other tissues. Arch Int Physiol Biochim. 1964 Jun;72(3):427–447. doi: 10.3109/13813456409065351. [DOI] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G. C., Frenkel A. W. Some Respiratory Characteristics of the Blue-Green Alga, Anabaena. Plant Physiol. 1953 Jan;28(1):63–69. doi: 10.1104/pp.28.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildon D. C., Rees T. A. Metabolism of Glucose-C by Anabaena cylindrica. Plant Physiol. 1965 Mar;40(2):332–335. doi: 10.1104/pp.40.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]



