Abstract
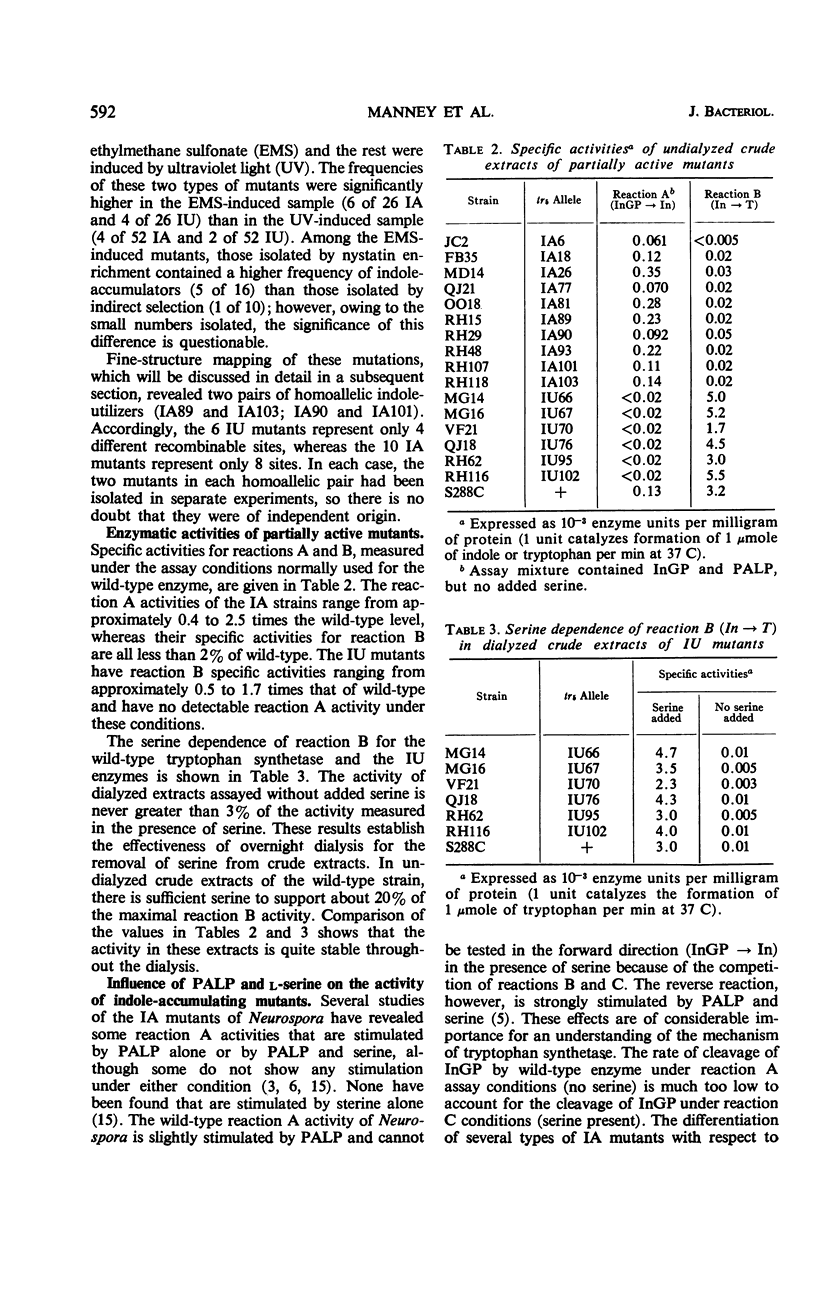
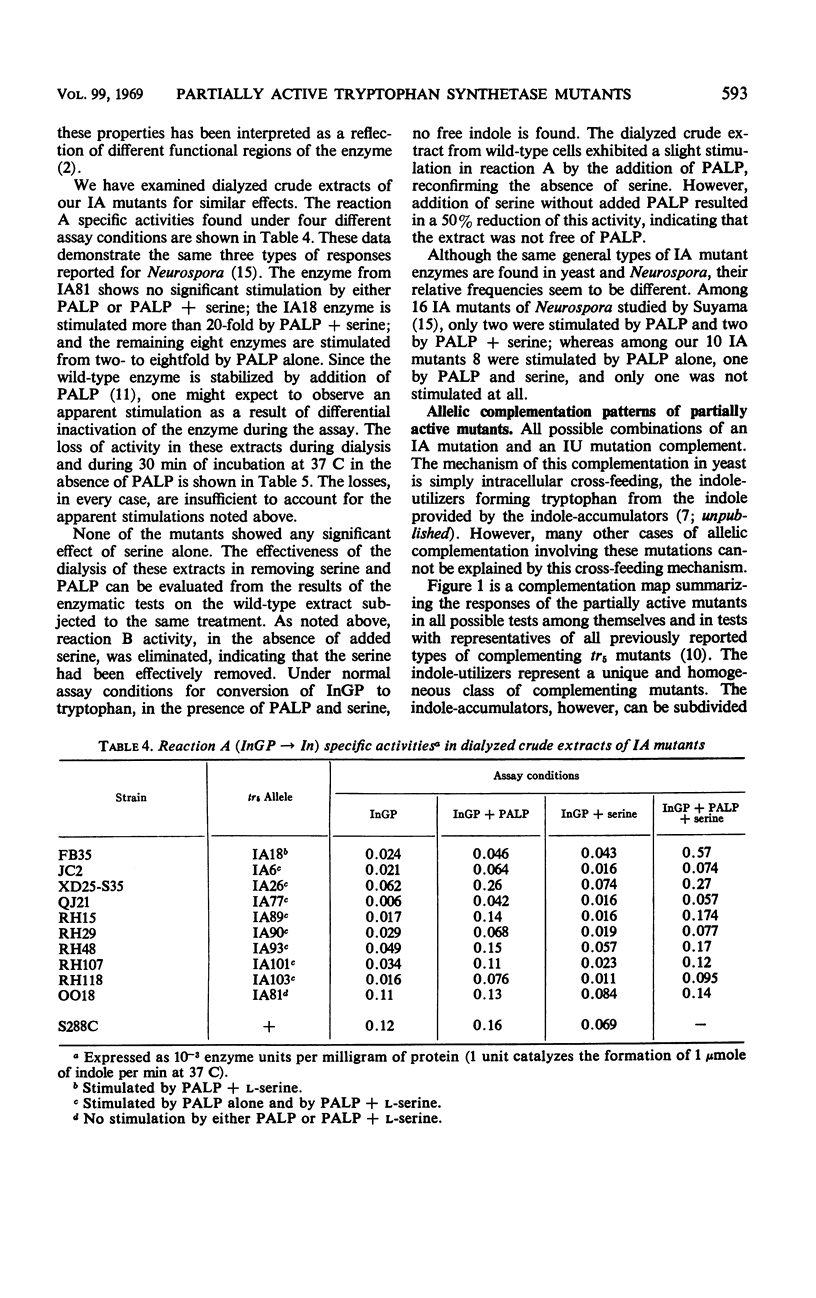
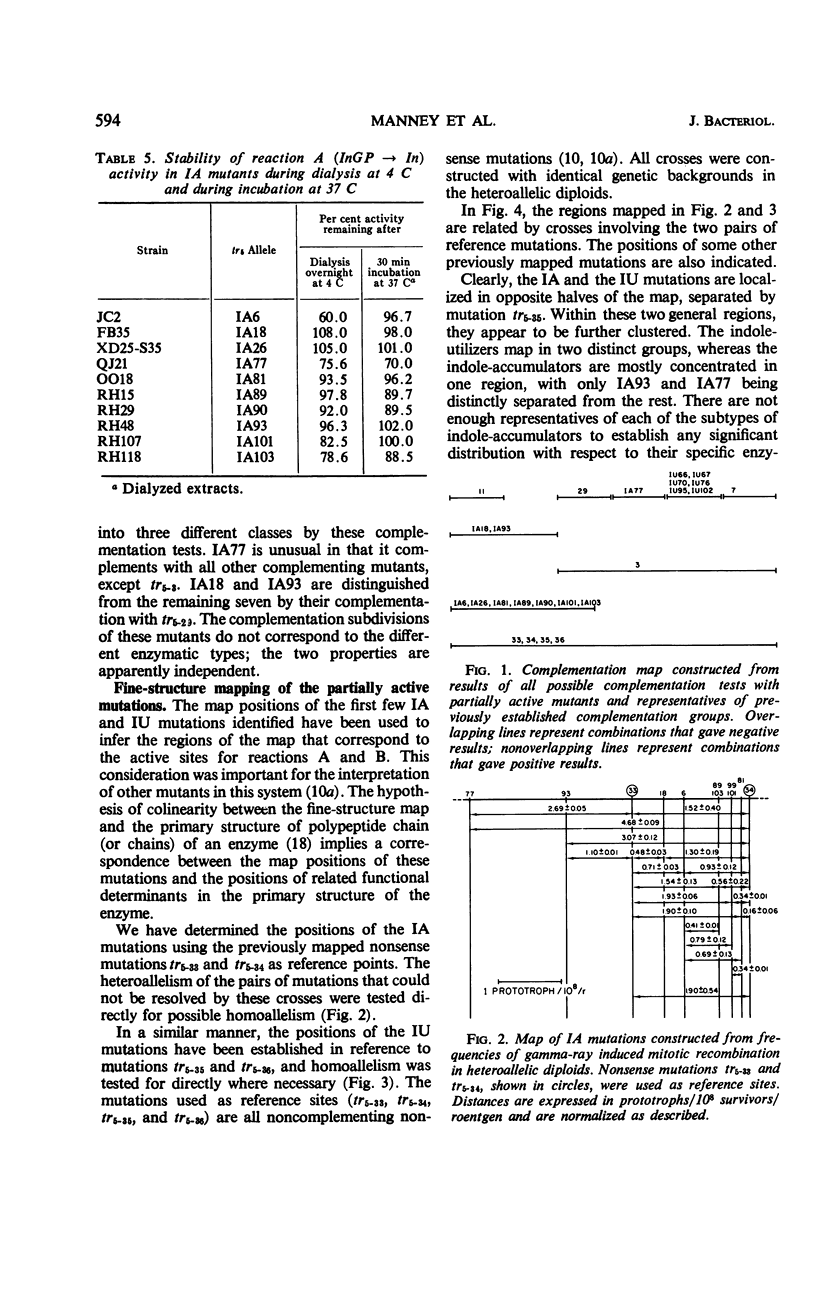
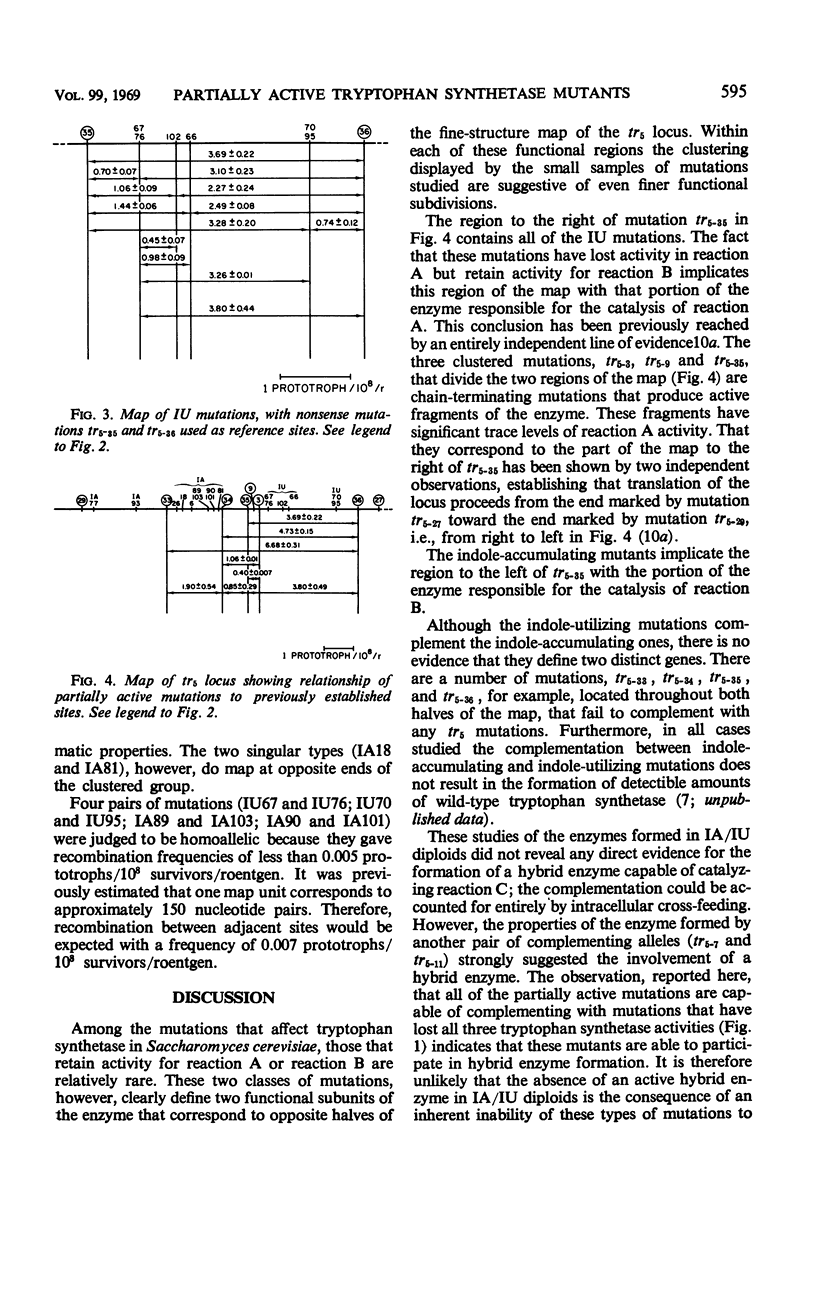
Approximately 20% of the tryptophan synthetase mutants (tr5) of Saccharomyces cerevisiae retain activity in one of the half reactions catalyzed by this enzyme and have been identified as indole-accumulating or indole-utilizing tr5 mutants by complementation tests. Ten indole-accumulating and six indole-utilizing mutants have been studied. For the half reactions they catalyze, these partially active mutants have from about one-half to twice the specific activities of the wild-type enzyme. Indole-accumulating mutant enzymes showed varying responses to pyridoxal phosphate and serine in the assay mixture. The partially active mutants were further characterized by their patterns of allelic complementation and their distribution on the fine-structure map of the locus. It was concluded that these mutants define two distinct functional regions of the tr5 locus, corresponding to the two half reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONNER D. M. CORRELATION OF THE GENE AND PROTEIN STRUCTURE. J Exp Zool. 1964 Oct;157:9–20. doi: 10.1002/jez.1401570106. [DOI] [PubMed] [Google Scholar]
- CARSIOTIS M., SUSKIND S. R. THE ROLE OF PYRIDOXAL PHOSPHATE IN THE ALDOLYTIC ACTIVITY OF TRYPTOPHAN SYNTHETASE FROM NEUROSPORA CRASSA. J Biol Chem. 1964 Dec;239:4227–4231. [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOSS J. A. Studies on the mechanism of the tryptophan synthetase reaction. Biochim Biophys Acta. 1962 Aug 13;62:279–293. doi: 10.1016/0006-3002(62)90041-0. [DOI] [PubMed] [Google Scholar]
- Demoss J. A., Bonner D. M. STUDIES ON NORMAL AND GENETICALLY ALTERED TRYPTOPHAN SYNTHETASE FROM NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1405–1412. doi: 10.1073/pnas.45.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntze W., Manney T. R. Two mechanisms of allelic complementation among tryptophan synthetase mutants of Saccharomyces cerevisiae. J Bacteriol. 1968 Dec;96(6):2085–2093. doi: 10.1128/jb.96.6.2085-2093.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. E., Creighton T. E., Baldwin R. L., Yanofsky C. Subunit structure of the tryptophan synthetase of Escherichia coli. J Mol Biol. 1966 Oct 28;21(1):71–82. doi: 10.1016/0022-2836(66)90080-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANNEY T. R. ACTION OF A SUPER-SUPPRESSOR IN YEAST IN RELATION TO ALLELIC MAPPING AND COMPLEMENTATION. Genetics. 1964 Jul;50:109–121. doi: 10.1093/genetics/50.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNEY T. R., MORTIMER R. K. ALLELIC MAPPING IN YEAST BY X-RAY-INDUCED MITOTIC REVERSION. Science. 1964 Feb 7;143(3606):581–583. doi: 10.1126/science.143.3606.581. [DOI] [PubMed] [Google Scholar]
- MOHLER W. C., SUSKIND S. R. The similar properties of tryptophan synthetase and a mutationally altered enzyme in Neurospora crassa. Biochim Biophys Acta. 1960 Sep 23;43:288–299. doi: 10.1016/0006-3002(60)90439-x. [DOI] [PubMed] [Google Scholar]
- Manney T. R. Evidence for chain termination by super-suppressible mutants in yeast. Genetics. 1968 Dec;60(4):719–733. doi: 10.1093/genetics/60.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R. Regulation of factors that influence the in vitro stability of tryptophan synthetase from yeast. J Bacteriol. 1968 Aug;96(2):403–408. doi: 10.1128/jb.96.2.403-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Crawford I. P. Purification and properties of the B component of Escherichia coli tryptophan synthetase. J Biol Chem. 1965 Dec;240(12):4801–4808. [PubMed] [Google Scholar]
- YANOFSKY C., CARLTON B. C., GUEST J. R., HELINSKI D. R., HENNING U. ON THE COLINEARITY OF GENE STRUCTURE AND PROTEIN STRUCTURE. Proc Natl Acad Sci U S A. 1964 Feb;51:266–272. doi: 10.1073/pnas.51.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., RACHMELER M. The exclusion of free indole as an intermediate in the biosynthesis of tryptophan in Neurospora crassa. Biochim Biophys Acta. 1958 Jun;28(3):640–641. doi: 10.1016/0006-3002(58)90533-x. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



