Abstract
For the act of membrane fusion, there are two competing, mutually exclusive molecular models that differ in the structure of the initial pore, the pathway for ionic continuity between formerly separated volumes. Because biological “fusion pores” can be as small as ionic channels or gap junctions, one model posits a proteinaceous initial fusion pore. Because biological fusion pore conductance varies widely, another model proposes a lipidic initial pore. We have found pore opening and flickering during the fusion of protein-free phospholipid vesicles with planar phospholipid bilayers. Fusion pore formation appears to follow the coalescence of contacting monolayers to create a zone of hemifusion where continuity between the two adherent membranes is lipidic, but not aqueous. Hypotonic stress, causing tension in the vesicle membrane, promotes complete fusion. Pores closed soon after opening (flickering), and the distribution of fusion pore conductance appears similar to the distribution of initial fusion pores in biological fusion. Because small flickering pores can form in the absence of protein, the existence of small pores in biological fusion cannot be an argument in support of models based on proteinaceous pores. Rather, these results support the model of a lipidic fusion pore developing within a hemifused contact site.
Keywords: membrane fusion, membranes, exocytosis, viral fusion
Membrane fusion is a ubiquitous event in eukaryotic cells, critical to organellar membrane trafficking, synaptic transmission, fertilization, enveloped viral infection, and myoblast syncytia formation. When biological membranes fuse, the first signal is an increase in the ionic conductivity between their contents (1, 2). In exocytosis and cell–cell fusion mediated by viral glycoproteins, fusion pores open quasi-abruptly to initial conductances varying between 30 and 1000 pS, which suggests pore diameters in the range of 1–7 nm (1–6). After opening, pores enter a phase in which their mean conductance does not vary substantially, the semistable phase (4, 5). Subsequently, there is a phase of rapid fusion–pore growth. To test whether initial fusion pores can be lipidic (7, 8) rather than proteinaceous (9, 10), we studied fusion of phospholipid bilayers.
In a number of electrophysiological studies on the fusion of phospholipid vesicles to planar phospholipid membranes, fusion has been demonstrated by membrane mixing with incorporation of channels from the vesicular to the planar membrane and by aqueous content mixing seen with fluorescence (11–17). Complete fusion of membranes is defined as the unification of the lipid bilayers of the membranes, preserving monolayer identity, concurrent with the mixing of the aqueous contents previously separated by two membranes. Hemifusion is defined as the coalescence of only the contacting membrane monolayers of the membranes, with the distal monolayers and aqueous contents separated (17). Complete fusion requires osmotic swelling of the adherent vesicle (11–13). It was originally thought that this osmotically driven fusion did not involve hemifusion (14), in contrast to the fusion of two planar bilayers (18). Recently (17) we found stable hemifusion intermediates between vesicles and planar bilayer membranes when the contacting leaflets consisted of lipids whose spontaneous curvature fits into the curvature of local connections between membranes (stalks) (19–21). In contrast, fusion pore formation and expansion depend on the lipid composition of the distal membrane monolayers. Lipids of a micelle-like spontaneous curvature, which matches the curvature of the pore edge, promote formation and expansion of a fusion pore when added to the distal leaflets. This suggests that the fusion pore in vesicle/planar bilayer fusion develops within the hemifusion diaphragm. To test the sequence of molecular events in fusion, we made simultaneous measurements of lipid dye mixing (membrane merger), aqueous dye mixing (content mixing), and electrical measurements of planar membrane conductance during the fusion of vesicles to planar bilayers. This allowed us to distinguish the merger of membrane leaflets, the formation of the fusion pore, and the release of vesicular contents. We report here flickering fusion pore formation between purely lipid bilayer membranes. A preliminary communication on this finding has been published in abstract form (43).
MATERIALS AND METHODS
Membranes and Solutions.
Planar bilayers (Fig. 1A) were formed across a hole in a Teflon partition by the Montal-Mueller technique, as previously described (17), from an n-hexane solution of asolectin [soybean lipids with neutral lipids removed (Avanti Polar Lipids)]. Giant lipid vesicles (2–20 μm) were formed from 70 weight % asolectin/20% ergosterol/10% rhodamine phosphatidylethanolamine as previously described (14). For osmotic stress, vesicles were formed in buffer B [200 mM calcein/10 mM Mes/5 mM n-propyl gallate, pH 6.5 (835 mOsm/kg)], and the planar bilayer was bathed by buffer A [400 mM KCl/20 mM CaCl2/10 mM Hepes/1 mM EDTA, pH 7.5 (760 mOsm/kg)]. In experiments with osmotically balanced liposomes, liposomes were formed in buffer B diluted 10 times with buffer A (resulting in a 780 mOsm/kg solution), and the planar bilayer was bathed by buffer A supplemented with 20 mM stachyose to achieve colloid osmotic equilibrium between internal content of liposomes and the medium bathing them (780 mOsm/kg). The channel-forming antibiotic nystatin was prepared as a stock solution in dimethyl sulfoxide (10 mg/ml) and then added to aqueous solutions with stirring before use. Nystatin was added to the liposome internal solution (10 μg/ml) before formation of liposomes to trap nystatin within liposomes. When nystatin was added to the buffer (50 μg/ml) in the same compartment of the chamber as liposomes, two-sided channels were formed in the vesicle membrane but not in the planar bilayer.
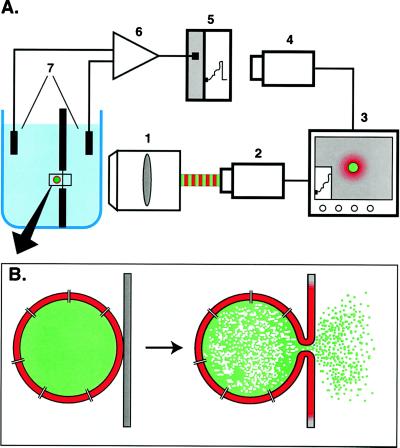
Figure 1.
Experimental system. (A) The chamber, similar to that described (14, 17), was placed in front of the long working distance objective (1) (Nikon, ×40, 0.5 numerical aperture). A custom-made dual-wavelength fluorescent imaging system was used to alternate the recording of the fluorescence of two dyes (42). The fluorescent image was projected onto an intensified video camera (2). Another camera (4) was focused on a chart recording (5) of transmembrane current, and this image was merged with the microscope image, providing the reference for synchronization (3) of fluorescence and electrical data, recorded on an optical disk recorder (not shown). A conventional voltage clamp amplifier (6) converted transmembrane current measured across Ag/AgCl electrodes (7), and current was also recorded on a modified digital–analog tape recorder (not shown). Individual lipid vesicles were placed on the planar membrane by using a glass micropipette (not shown) filled with a liposome suspension. (B) Schematic diagram of the fusion of a vesicle to a planar membrane. Fusion can be detected as membrane dye spread, release of water-soluble dye, a conductance increase because of channel incorporation, or as the increase in capacitance proportional to the increase in membrane area.
Simultaneous Electrical and Fluorescence Measurements.
After formation of the planar bilayer, a suspension of liposomes in a pipette was injected into the buffer near the membrane. One liposome was selected by size and placed on the center of the membrane by manipulation of the pipette. Thirty millivolts transmembrane potential was applied across the membrane, and recordings of the membrane conductance, lipid dye fluorescence, and aqueous dye fluorescence were made as described in the legend (Fig. 1A).
For admittance measurements, liposomes were prepared and pipetted onto planar bilayers as described above, except no nystatin was used internally or in bilayer chamber. A 100-mV (peak-to-peak), 500-Hz sine wave was applied to the planar membrane on top of a 30 mV holding potential (cis side positive). Most of the capacitive current was compensated by a modified voltage clamp [one capacitor was added to the head stage of an Axopatch 200 (Axon Instruments, Foster City, CA)]. The output of the amplifier was digitized and analyzed by in-house lock-in software (Browse, available on request) and stored as the in-phase and out-of-phase components of the complex admittance and the DC conductance, all with 4-ms resolution (average of pairs of sine waves). Ten-point digital averaging and decimation were used to improve the signal-to-noise ratio, resulting in 40 ms per point. By using Browse, fusion pores were calculated as described (22, 23), with either a combination of the in-phase and out-of-phase components or only the in-phase component, because in many cases the capacitance of planar bilayer became unstable after hemifusion. Comparison of the in-phase component and the DC conductance was used to verify that fusion pores did not represent electrical leakage across the bilayer.
RESULTS
To detect the different aspects of membrane fusion, vesicles were prepared to carry three different markers: lipid dye, aqueous dye, and ionic channels. After individual giant (2–20 μm) vesicles were pipetted onto planar membranes (cis side), fusion was detected by the spread of lipid dye, release of water-soluble dye, and an increase in membrane conductance (Fig. 1B). Complete fusion, defined as both the coalescence of the vesicular membrane into the planar membrane and the release of vesicular contents into the trans compartment, is detected as a change in all three markers and is marked in time as a large step in membrane conductance (12). Hemifusion, defined as the merger of contacting, but not distal, leaflets of membranes, is seen as lipid dye mixing with no aqueous dye dilution or conductance changes. Fusion pore formation, defined as a narrow aqueous pathway connecting the interior of the vesicle to the trans compartment, was seen as a change in membrane conductance together with aqueous dye dilution. Simultaneous measurements of these three parameters allowed us to discard experiments of vesicle rupture or leakage, seen as aqueous dye dilution with no electrical changes or lipid dye mixing. Such leakage was observed both before and after liposome binding to the planar membrane.
As expected from previous publications, only a fraction of the encounters between a vesicle and the phospholipid membrane resulted in any sign of fusion, and complete fusion required osmotic tension to swell the vesicle (12–16). Lipid dye transfer was detected in only 85 of 415 experiments. In the other 330 experiments, either none of the three signals was detected at all (n = 273) or conductance changes were seen in the absence of lipid dye transfer, or aqueous dye diminished without either lipid dye transfer or conductance changes (n = 57), presumably because of leakage cis from the vesicle. Because of high electrical noise or premature lysis of the liposomes in some experiments, we were able to analyze in detail only 68 of the 85 experiments with lipid dye transferred. In 75% of these 68 experiments, the first detected signal was an increase in the fluorescence of the lipid dye. Because in these 51 experiments no aqueous dye release or any conductance increase was observed for some time during and after lipid dye mixing, we interpret this union of results to indicate merging of only the contacting membrane monolayers, hemifusion in these experiments (Fig. 2A). Hemifusion between vesicles and planar membranes may be reversible or restricted, rather than all-or-none, because in many cases lipid dye redistribution occurred in more than one stage before any conductance changes. This restriction to lipid flux is intriguing and may explain why conductance steps were detected before detectable lipid dye transfer in 8 of the 68 analyzed experiments, and no dye redistribution was seen in 9 of these 68 experiments. Finally, lipid dye redistribution ceased, presumably because of complete loss of lipid dye from the outer leaflet of the vesicle. In hemifusion, the inner leaflet of the vesicle should not mix with the trans leaflet of the planar bilayer. In agreement with this, lipid dye fluorescence in the liposome was seen after hemifusion was completed (Fig. 2A, last panel).
Figure 2.
Hemifusion and fusion pore formation in fusion of individual liposomes with the planar membrane. Fluorescent images were obtained with membrane dye rhodamine phosphatidylethanolamine (red) and aqueous dye calcein (green) filter sets. Scale bars in all panels are 10 μm for images, and 30 pS and 10 s for electrical recordings. Blue lines show the frame position on a time scale represented by the recording of transmembrane current. Throughout, transmembrane voltage was +30 mV with respect to the trans chamber. All records begin from the moment of vesicle placement onto the cis side of the planar membrane. (A) Hemifusion of a liposome in osmotic balance with external medium. Membrane dye (red) spread to the planar membrane radially from the liposome (panel 3) and then dispersed throughout the planar film. This demonstrates hemifusion of the vesicle with the planar membrane. The inner leaflet of the liposome would not mix in hemifusion: correspondingly, liposome membrane dye fluorescence remains bright despite the flash of membrane dye in panels 3–5. Neither calcein fluorescence nor membrane conductance changed during hemifusion. (B) Transient fusion pores in an osmotically balanced liposome observed after hemifusion of the liposome with the planar membrane. Hemifusion was detected as in A approximately 10 s before panel 2. A significant decrease in content calcein fluorescence (compare green panels 2–3 and 4–5) correlates with spikes of transmembrane current (fusion pore flickering). A second rhodamine flash (panel 6), more intensive then the first, presumably corresponds to an expansion of the zone of hemifusion. More content release is seen with continued fusion pore flickering. As in A, membrane dye is still visible in the liposome (panel 7) after the diffusion of released membrane dye over the planar membrane, more evidence for hemifusion. In control experiments neither liposomes without nystatin nor nystatin added without liposomes produced similar spikes. (C) Fusion of an osmotically stressed liposome with the planar membrane. (1–2) Hemifusion, seen as a rhodamine lipid dye flash (red) with no loss of calcein (green); (3–4) spikes of transmembrane current (transient fusion pore formation) with loss of liposome content calcein (green), continuing up through (5–6) expansion of hemifusion diaphragm. Finally the vesicle undergoes complete fusion, resulting in a large, off-scale increase in the conductance of planar bilayer (greater than 200 pS). This conductance then rapidly decreases toward baseline as a result of dilution and disassembling of nystatin–sterol channel complexes in the fused membranes. (7) At this stage inner and outer leaflet lipid dye (red) and internal content (green) have completely redistributed.
Subsequent to hemifusion, fusion pore formation was detected by concomitant release of aqueous dye and conductance increments. For instance, in Fig. 2B, following an initial mixing of lipid dye seen as an increase in red membrane dye fluorescence between panels 1 and 2 (hemifusion), the first two spikes of transmembrane current clearly correlated with the release of green aqueous dye from the vesicle, indicating formation of a fusion pore connecting the two membranes. We saw fusion pore formation following hemifusion in 76% of the 51 hemifusion events. Usually pores were detected only one to two times per experiment, but on occasion long trains of fusion pores were recorded (Fig. 2B). The sequence of lipid dye mixing followed by aqueous dye release and transient fluctuations of membrane conductance was similar for both osmotically stressed and osmotically balanced liposomes. However, only 8% of analyzed experiments with balanced liposomes showed large jumps in conductance. When liposomes were hypotonically stressed, 32 of 43 analyzed experiments showed dye redistribution before conductance changes. The pattern of spikes followed by a large conductance jump occurred 18 times of the 43 stressed cases, corresponding to complete fusion. The large conductance jump occurred without spikes preceding it in 9 stressed cases. Spikes without jumps were seen in 15 of 16 remaining experiments. Conductance then dissipated (Fig. 2C) as nystatin and sterol were diluted by diffusion, in agreement with the sterol and concentration requirements for the one-sided action of nystatin (24). The flickering conductance spikes, at higher time resolution, are varied in amplitude and lifetime within each liposome/membrane pair (Fig. 3 A–C).
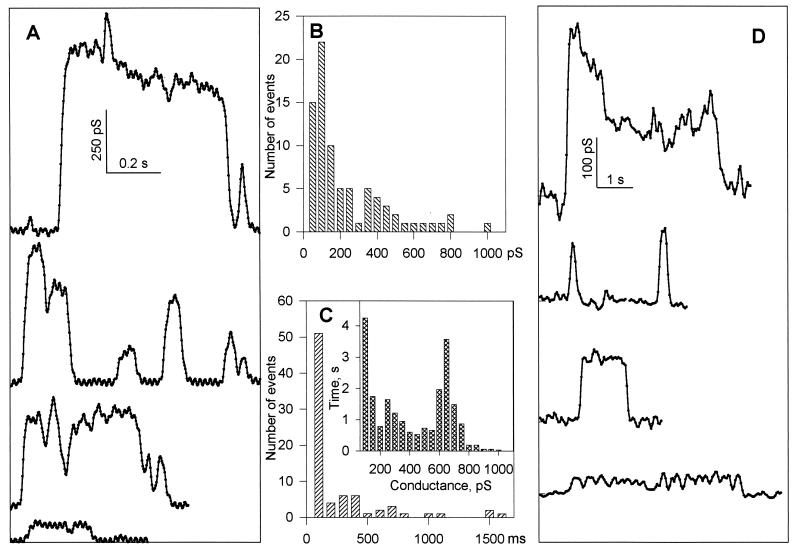
Figure 3.
Conductance characteristics of fusion pores in phospholipid membranes. (A) Time-resolved fusion pore conductance in experiments with nystatin-permeabilized liposomes. Four independent short segments of membrane current, digitized at 400 Hz on a computer, are presented (after 10 points averaging and decimation to reduce noise) as typical examples of fusion pores. Conductance was estimated for the fusion pore in series with a 4.7-nS liposome (average liposome conductance). (B) Frequency histogram of maximum pore conductances. As in A, conductance was estimated for the fusion pore in series with a 4.7-nS liposome. (C) Histogram of pore lifetimes. Pore lifetimes were determined by analysis of the 10-point averaged and decimated data, by picking the first and last point of transients continuously above the noise of the baseline for greater than 100 ms. (Inset) Integrated histogram of pore conductance (see ref. 5). The time axis is defined as the time that a pore spends in a state within a given conductance interval, combining all fusion pores detected above noise (50 pS). (A–C) Nystatin-containing liposomes. (D) Time-resolved fusion pore conductance calculated from admittance measurements in nystatin-containing liposomes.
The use of nystatin ionic channels allowed us to use the direct measurement of planar bilayer conductance to monitor fusion, as in previous work (14, 15). However, it was possible that the presence of nystatin affected the fusion process. To test whether fusion pore formation occurs in the complete absence of protein or peptides and to measure the pore as a conductance in series with the capacitance of the vesicle, we used the admittance measurement technique (22, 25) with purely phospholipid vesicles, devoid of nystatin. (In 50 of 81 experiments, no signals were detected at all, and in 16 experiments either aqueous dye release occurred alone or membranes became leaky.) In 15 experiments we observed lipid dye redistribution. In three of these experiments, fusion pores were detected in the presence of transient transbilayer conductances (leaky fusion). In 7 of 15 experiments showing hemifusion events, long lasting fusion pores were detected (again) as for nystatin-containing liposomes, after hemifusion of liposomes with planar membranes and in the absence of any transbilayer conductance changes (Fig. 3D). These fusion pores had conductances within the distribution of conductance changes observed for nystatin-containing liposomes. Time-resolved records of pores obtained with either of these methods were similar (compare Fig. 3 A and D). The difference in amplitudes of pores formed in nystatin-containing and nystatin-free liposomes may reflect some effect of nystatin on fusion. Nonetheless, taken together with the fluorescence data, these capacitance spikes can only be interpreted as the formation of an aqueous fusion pore allowing ionic continuity between the inner volume of vesicle and the solution bathing the opposite side of the membrane to which vesicles were added (trans compartment).
DISCUSSION
On the basis of these data, we can present the sequence of contact, hemifusion, fusion pore formation, and expansion in the fusion of protein-free phospholipid membranes (Fig. 4). The spikes of membrane current and the transients in membrane admittance described above reflect the opening of transient fusion pores through which the high conductance of the permeabilized vesicle membrane shunted the low conductance of the planar bilayer. Pores of similar conductances and lifetimes develop in single planar lipid bilayers under high electric fields (26). These electrically induced lipidic pores flicker until their final expansion, which can be described as a pore reaching some critical radius determined by a balance between the linear tension of a pore edge and bilayer surface tension. The evolution of pores developed in the hemifusion diaphragm should be controlled by the same physical forces.
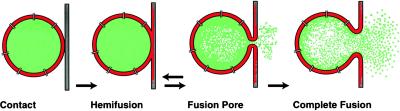
Figure 4.
Schematic diagram of sequential steps in phospholipid membrane fusion. As a first step the outer monolayer of a phospholipid vesicle coalesces with the contacting monolayer of the planar phospholipid bilayer membrane forming a trilaminar structure (hemifusion). A small fusion pore is formed in this area. Once formed, the fusion pore can close again or expand to some critical size when further expansion becomes irreversible.
Hemifusion has been described for the fusion of two planar lipid bilayers (20) and for vesicle–planar bilayer fusion (17). Most recently, hemifusion has been documented for vesicle–vesicle fusion (ref. 27; see also refs. 28 and 29). Lee and Lentz (27) compared the kinetics of lipid mixing of outer vs. inner leaflets of sonicated phosphatidylcholine vesicles along with changes in the molecular sieving of vesicle contents, all induced by polyethylene glycol. In their suspension of fusing liposomes, the hemifusion signal preceded that of complete fusion. Importantly, the earliest observable in these experiments has been a fast and short-term increase in the transfer between vesicles of the smallest aqueous marker used, the proton. This finding has been interpreted as an indication of the transient opening of small fusion pores. In vesicle suspension, a population of lipid bilayers simultaneously comes in contact, fuses, and exchanges markers. Also, for vesicle–vesicle fusion the stress of apposition induces an increased internal pressure and leads to complete fusion; vesicle–planar bilayer fusion only goes to completion when intravesicular pressure is applied osmotically (11, 12). In the present work we follow the fusion pathway for an individual phospholipid vesicle fusing with planar bilayer. We can directly observe the sequence of (i) hemifusion, (ii) flickering fusion pores, and then (iii) an expanding fusion pore for single fusion events. Thus the fusion of lipid bilayers in different experimental systems proceeds via similar membrane rearrangements.
Whereas there are quantitative variations in the sizes of viral and exocytotic fusion pores, fusion pores in biological fusion appear to share some features with those in phospholipid vesicle to planar bilayer fusion. The mean value of our fusion pore conductances (206 pS, nystatin experiments) for liposome–planar lipid bilayer fusion is close to the mean values of initial fusion pores that were reported for exocytosis in neutrophils and in mast cells [150 pS (6) and 330 pS (3), respectively], and their frequency distributions are similar. Thus there is no need to invoke proteinaceous pores in biological fusion.
A priori, then, there is nothing to rule out a lipidic nature of biological fusion. This view is supported by recent work showing a specific lipid dependence for exocytosis (30–33) and a lipid-dependent stage for the fusion of living membranes that is identical with that of purely phospholipid membranes (34–38). When biological membrane fusion is blocked by adding lipids of positive intrinsic curvature such as lysophosphatidylcholine, fusogenic proteins still change their conformation when triggered by even brief pulses of the appropriate ligands: calcium or pH (35–37). They can remain in this triggered conformation until the lipid block is lifted by either removing added lipid or adding lipid of the opposite curvature (36). Fusion ensues, even if the triggering conditions are no longer present. Changing lipid composition is enough to release the block so that fusion goes to completion. It is as if protein conformational change can do a finite amount of work and lead to a finite reduction in the energy barrier for fusion, and lipid composition sets that energy barrier. It is intriguing that the particular lipid that forms during exocytotic priming (30–33), phosphatidylinositol 4,5-bisphosphate (PIP2), should have profound positive intrinsic curvature judging by its structure. Because lipids of positive intrinsic curvature block merger of membranes with fusogenic proteins already in an activated state, PIP2 may do the same. A subsequent reaction such as Ca2+-dependent modification or removal of PIP2 or even direct calcium binding to the lipid could reverse the curvature of the monolayers and help fusion to continue to completion. That is, PIP2 may act, in part, as a fusion clamp to build up a ready-release pool, in addition to its roles in signal transduction (33).
Both hemifusion and transient, flicker fusion (demonstrated here for the fusion of purely lipid membranes) have been demonstrated for the fusion of natural membranes (8, 39–41). It seems logical that the evolution of fusion machinery in living organisms was probably based on regulation of existing physicochemical mechanisms for fusion, rather then on the de novo invention of some protein-driven machine operating on different principles. If this is correct, then the role of protein in multistep, in vivo fusion mechanisms may be limited to the creation of the required prefusion configuration of two bilayers, with participation of fusogenic proteins to reduce the energy barriers for the pivotal stages of membrane coalescence and pore formation. Fusion-inhibiting proteins may act by increasing these same barriers. This is consistent with the hypothesis that biological fusion, although controlled and mediated by specialized proteins, proceeds via lipid-dependent stages that involve the same rearrangement of membrane lipid as the fusion of purely lipidic bilayers.
ABBREVIATION
- PIP2
phosphatidylinositol 4,5-bisphosphate
References
- 1.Tse F W, Iwata A, Almers W J. Cell Biol. 1993;121:543–552. doi: 10.1083/jcb.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerberg J, Blumenthal R, Curran M, Sarkar D, Morris S. J Cell Biol. 1994;127:1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spruce A E, Breckenridge L J, Lee A K, Almers W. Neuron. 1990;4:643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- 4.Nanavati C, Markin V S, Oberhauser A F, Fernandez J M. Biophys J. 1992;63:1118–1132. doi: 10.1016/S0006-3495(92)81679-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran M J, Cohen F S, Chandler D E, Munson P J, Zimmerberg J. J Membr Biol. 1993;133:61–75. doi: 10.1007/BF00231878. [DOI] [PubMed] [Google Scholar]
- 6.Lollike K, Borregaard N, Lindau M. J Cell Biol. 1995;129:99–104. doi: 10.1083/jcb.129.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monck J R, Alvarez de Toledo G, Fernandez J M. Proc Natl Acad Sci USA. 1990;87:7804–7808. doi: 10.1073/pnas.87.20.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monck J R, Fernandez J M. J Cell Biol. 1992;119:1395–1404. doi: 10.1083/jcb.119.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almers W, Tse F W. Neuron. 1990;4:813–818. doi: 10.1016/0896-6273(90)90134-2. [DOI] [PubMed] [Google Scholar]
- 10.Lindau M, Almers W. Curr Opin Cell Biol. 1995;7:509–517. doi: 10.1016/0955-0674(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerberg J, Cohen F S, Finkelstein A J. J Gen Physiol. 1980;75:241–250. doi: 10.1085/jgp.75.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen F S, Zimmerberg J, Finkelstein A J. J Gen Physiol. 1980;75:251–270. doi: 10.1085/jgp.75.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen F S, Akabas M H, Zimmerberg J, Finkelstein A. J Cell Biol. 1984;98:1054–1062. doi: 10.1083/jcb.98.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niles W D, Cohen F S. J Gen Physiol. 1987;90:703–735. doi: 10.1085/jgp.90.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodbury D J, Hall J E. Biophys J. 1988;54:1053–1063. doi: 10.1016/S0006-3495(88)83042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perin M S, MacDonald R C. J Membr Biol. 1989;109:221–232. doi: 10.1007/BF01870279. [DOI] [PubMed] [Google Scholar]
- 17.Chernomordik L, Chanturiya A, Green J, Zimmerberg J. Biophys J. 1995;69:922–929. doi: 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neher E. Biochim Biophys Acta. 1974;373:327–336. doi: 10.1016/0005-2736(74)90012-1. [DOI] [PubMed] [Google Scholar]
- 19.Kozlov, M. M. & Markin, V. S. (1983) Biofizika (Engl. Transl.) 28, 255–261.
- 20.Chernomordik L V, Melikyan G B, Chizmadzhev Y A. Biochim Biophys Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 21.Siegel D P. Biophys J. 1993;65:2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindau M. Q Rev Biophys. 1991;24:75–101. doi: 10.1017/s0033583500003279. [DOI] [PubMed] [Google Scholar]
- 23.Plonsky I, Zimmerberg J. J Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinberg M E, Finkelstein A. J Membr Biol. 1984;80:257–269. doi: 10.1007/BF01868444. [DOI] [PubMed] [Google Scholar]
- 25.Neher E, Marty A. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chernomordik L V, Sukharev S I, Popov S V, Pastushenko V F, Sokirko A V, Abidor I G, Chizmadzhev Y A. Biochim Biophys Acta. 1987;902:360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee J K, Lentz B R. Biochemistry. 1997;36:6251–6259. doi: 10.1021/bi970404c. [DOI] [PubMed] [Google Scholar]
- 28.Duzgunes N, Straubinger R M, Baldwin P A, Friend D S, Papahadjopoulos D. Biochemistry. 1985;24:3091–3098. doi: 10.1021/bi00334a004. [DOI] [PubMed] [Google Scholar]
- 29.Ellens H, Bentz J, Szoka F C. Biochemistry. 1986;25:285–294. doi: 10.1021/bi00350a001. [DOI] [PubMed] [Google Scholar]
- 30.Eberhard D A, Cooper C L, Low M G, Holz R W. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay J C, Fisette P L, Jenkins G H, Fukami K, Takenawa T, Anderson R A, Martin T F. Nature (London) 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 32.Hay J C, Martin T F. Nature (London) 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 33.Martin T F. Trends Cell Biol. 1997;7:271–276. doi: 10.1016/S0962-8924(97)01060-X. [DOI] [PubMed] [Google Scholar]
- 34.Chernomordik L V, Vogel S S, Sokoloff A, Onaran H O, Leikina E A, Zimmerberg J. FEBS Lett. 1993;318:71–76. doi: 10.1016/0014-5793(93)81330-3. [DOI] [PubMed] [Google Scholar]
- 35.Vogel S S, Leikina E A, Chernomordik L V. J Biol Chem. 1993;268:25764–25768. [PubMed] [Google Scholar]
- 36.Chernomordik L V, Leikina E, Frolov V, Bronk P, Zimmerberg J. J Cell Biol. 1997;136:81–94. doi: 10.1083/jcb.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerberg J, Vogel S, Walley T, Plonsky I, Sokoloff A, Chanturiya A, Chernomordik L V. Cold Spring Harbor Symp Quant Biol. 1995;60:589–599. doi: 10.1101/sqb.1995.060.01.063. [DOI] [PubMed] [Google Scholar]
- 38.Chernomordik L, Kozlov M, Zimmerberg J. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- 39.Song L Y, Ahkong Q F, Georgescauld D, Lucy J A. Biochim Biophys Acta. 1991;1065:54–62. doi: 10.1016/0005-2736(91)90010-6. [DOI] [PubMed] [Google Scholar]
- 40.Kemble G W, Danieli T, White J M. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 41.Melikyan G B, White J M, Cohen F S. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanturiya A N. J Fluoresc. 1996;6:103–106. doi: 10.1007/BF00732049. [DOI] [PubMed] [Google Scholar]
- 43.Chanturiya A N, Chernomordik L V, Zimmerberg J. Biophys J. 1995;68:118. doi: 10.1016/S0006-3495(95)79966-0. (abstr.). [DOI] [PMC free article] [PubMed] [Google Scholar]