Abstract
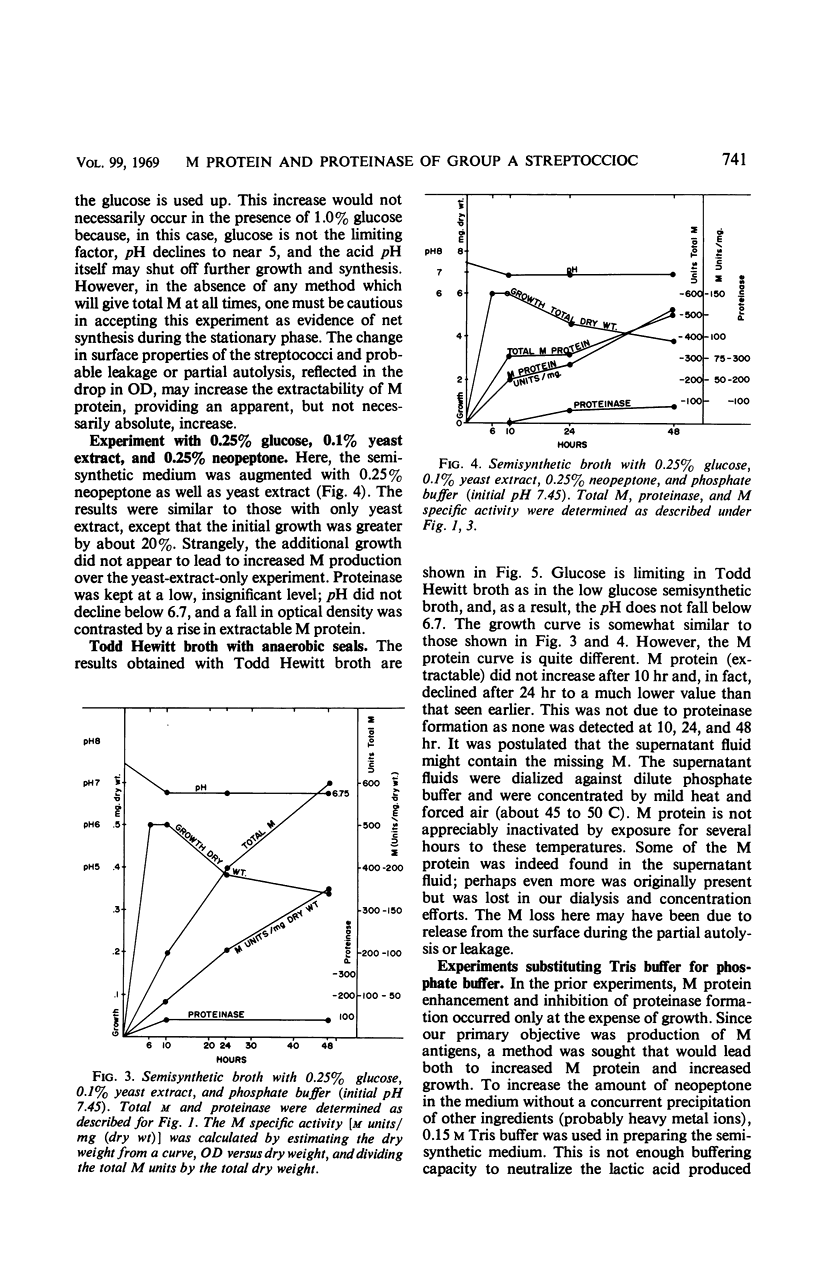
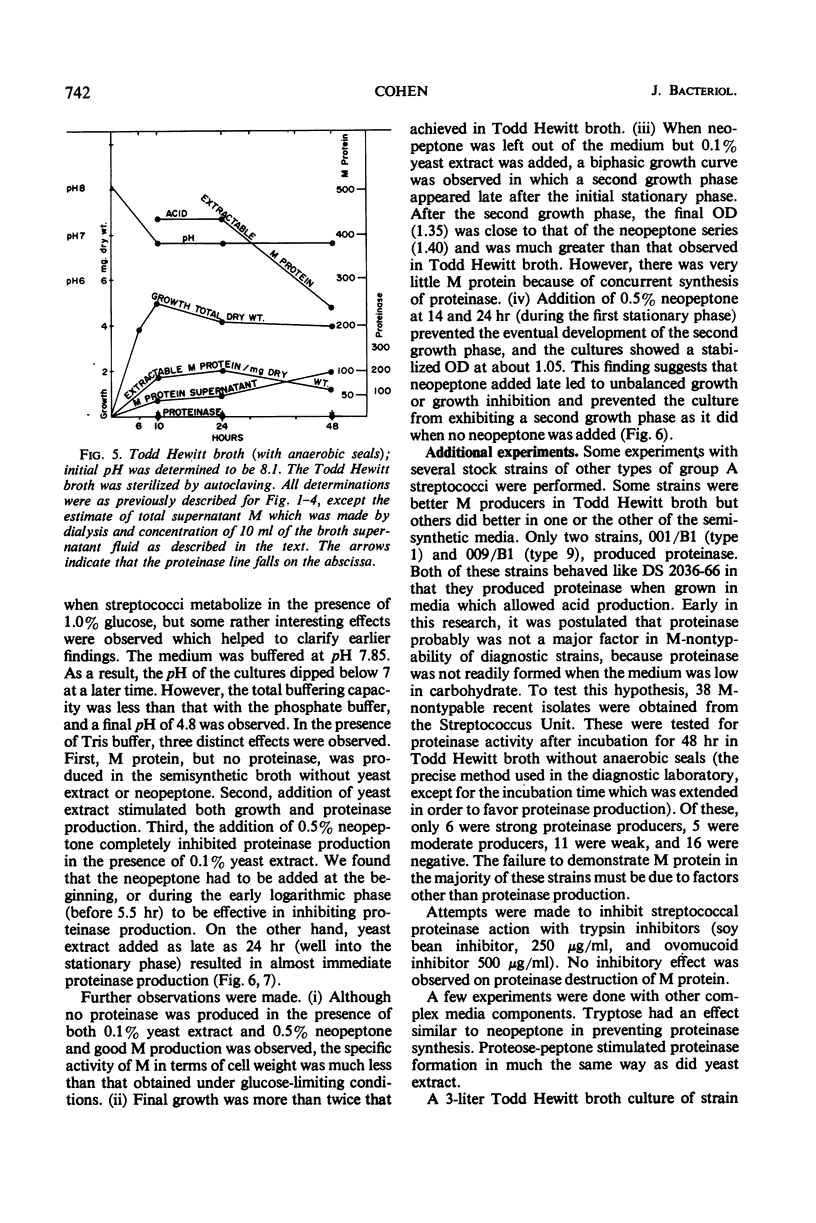
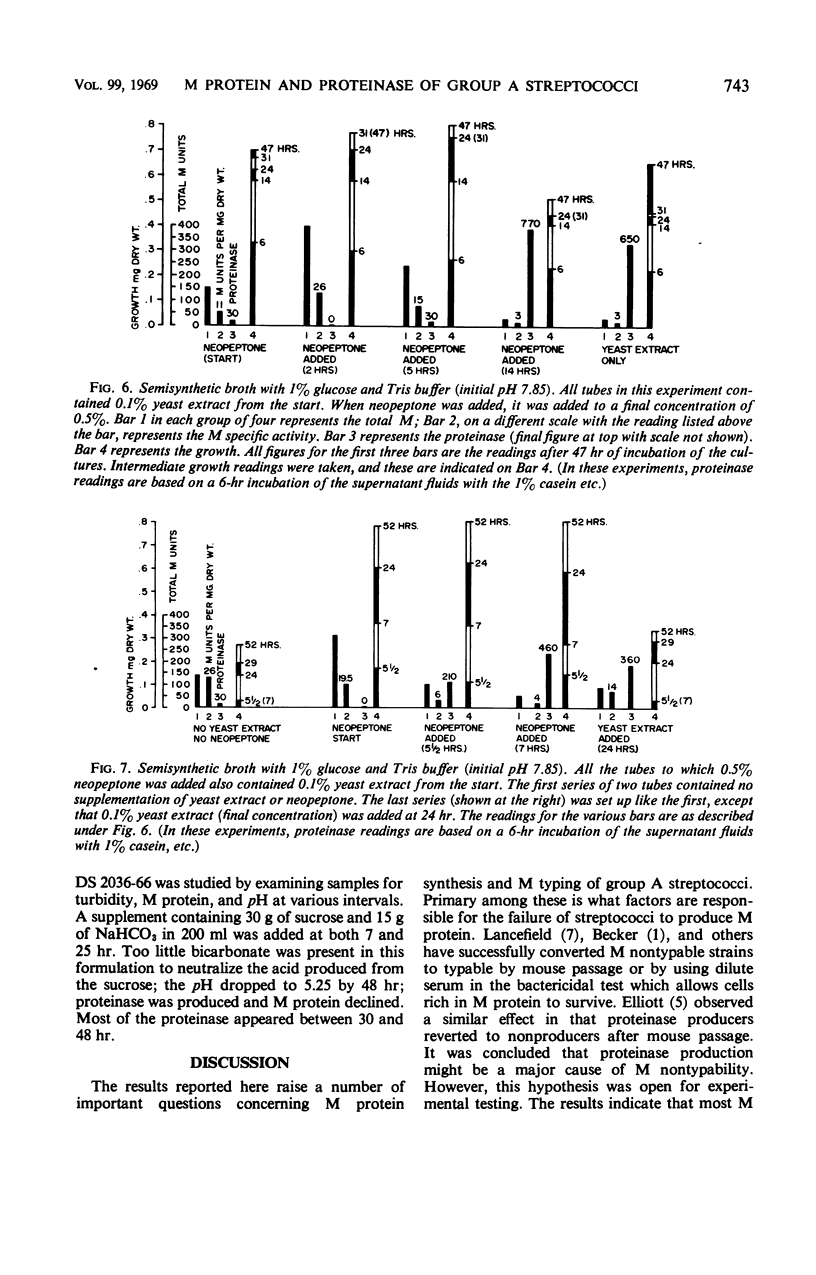
The effects of pH, yeast extract, and neopeptone on the production of extracellular proteinase and M protein by group A streptococci were studied with a type 1 strain capable of producing both M protein and proteinase. The strain DS 2036-66 grew moderately well in a semisynthetic broth. M protein was produced without adding peptides to the medium. When added to a medium with 1% glucose, yeast extract (0.1%) was found to stimulate both growth and proteinase formation. Limiting the glucose to 0.25% prevented a drop in pH below 6.7 and prevented proteinase formation. Although less growth occurred with limited glucose, M protein of high specific activity was produced with an actual increase in acid-extractable M protein during the stationary phase of growth. When the medium was buffered at pH 7.85 with tris(hydroxymethyl)aminomethane buffer, 0.5% neopeptone prevented proteinase formation. This was true even in the presence of 1% glucose and 0.1% yeast extract, which resulted in a fall in pH to about 4.8 by 48 hr. Growth was greater than in Todd Hewitt broth, but the specific activity of M protein was considerably less than that found in the medium with glucose limited to 0.25%. Neopeptone was found to have little direct action on crude streptococcal proteinase. Instead, the evidence suggested that neopeptone somehow prevents proteinase elaboration. Yeast extract, on the other hand, appears to stimulate proteinase elaboration. To prevent proteinase formation, neopeptone must be added early, during the logarithmic phase of growth or at the start. In contrast, when yeast extract was added as late as 24 hr, it resulted in the elaboration of extracellular proteinase and in the decline of M protein. When 38 M nontypable strains from the diagnostic laboratory were tested for proteinase activity under conditions similar to those used in the diagnostic laboratory, only six produced much proteinase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D. EFFECT OF ANTIBIOTICS AND INHIBITORS ON M PROTEIN SYNTHESIS. J Bacteriol. 1963 Mar;85:527–531. doi: 10.1128/jb.85.3.527-531.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. G. Enhancing effect of type specific antistreptococcal antibodies on emergence of streptococci rich in M-protein. Proc Soc Exp Biol Med. 1967 Jan;124(1):331–335. doi: 10.3181/00379727-124-31736. [DOI] [PubMed] [Google Scholar]
- Cohen J. O., Pine L. Quantitative aspects of the M protein capillary precipitin test. Appl Microbiol. 1968 Jan;16(1):122–127. doi: 10.1128/am.16.1.122-127.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. C., Karush F., Rudd J. H. Synthesis of M protein by group A hemolytic streptococci in completely synthetic media during steady-state growth. J Bacteriol. 1968 Jan;95(1):162–168. doi: 10.1128/jb.95.1.162-168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT S. D. The crystallization and serological differentiation of a streptococcal proteinase and its precursor. J Exp Med. 1950 Sep;92(3):201–218. doi: 10.1084/jem.92.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PINE L., WATSON S. J. Evaluation of an isolation and maintenance medium for Actinomyces species and related organisms. J Lab Clin Med. 1959 Jul;54(1):107–114. [PubMed] [Google Scholar]



