Abstract
Deletions of all or part of chromosome 10 are the most common genetic alterations in high-grade gliomas. The PTEN gene (also called MMAC1 and TEP1) maps to chromosome region 10q23 and has been implicated as a target of alteration in gliomas and also in other cancers such as those of the breast, prostate, and kidney. Here we sought to provide a functional test of its candidacy as a growth suppressor in glioma cells. We used a combination of Northern blot analysis, protein truncation assays, and sequence analysis to determine the types and frequency of PTEN mutations in glioma cell lines so that we could define appropriate recipients to assess the growth suppressive function of PTEN by gene transfer. Introduction of wild-type PTEN into glioma cells containing endogenous mutant alleles caused growth suppression, but was without effect in cells containing endogenous wild-type PTEN. The ectopic expression of PTEN alleles, which carried mutations found in primary tumors and have been shown or are expected to inactivate its phosphatase activity, caused little growth suppression. These data strongly suggest that PTEN is a protein phosphatase that exhibits functional and specific growth-suppressing activity.
The best characterized genetic alterations found in the malignant progression of human gliomas are inactivation of the genes for p53, p16, and RB as well as amplification of CDK4 and the epidermal growth factor receptor genes (for reviews see refs. 1–5). However, the most common genetic alteration is loss of heterozygosity on chromosome 10, which occurs late in tumor development and at a frequency of ≈70–90% (6–8). Recently, a candidate tumor suppressor gene, PTEN (9) [also known as MMAC1 (10) or TEP1 (11)] was mapped to 10q23 and shown to be mutated in 17–24% of xenografted and primary glioblastomas, 14% of breast cancer samples, and 25% of kidney carcinomas (10). Moreover, the mutation frequency in established cell lines of these tumor types is somewhat higher. In addition to this predicted involvement in sporadic cancer, germ-line PTEN mutations also have been detected in two autosomal dominant disorders, Cowden disease (12, 13), a syndrome that confers an elevated risk for tumors of the breast, thyroid, and skin, and Bannayan–Zonana syndrome (14), a condition characterized by macrocephaly, lipomas, intestinal hamartomatous polyps, vascular malformations, and some skin disorders.
The predicted protein product of the PTEN gene has an amino terminal domain with extensive homology to tensin, a protein that interacts with actin filaments at focal adhesions, and with auxilin, a protein involved in synaptic vesicle transport, as well as a domain with perfect homology to protein tyrosine phosphatases (9, 10). Mutations of PTEN in tumors, its cytoplasmic localization (11), and its intrinsic phosphatase activity (11, 15) suggest that its activity may be important in some aspect of tumor progression, possibly to counteract the oncogenic effect of a specific protein tyrosine kinase. PTEN also is rapidly down-regulated by type β transforming growth factor in cells sensitive to its cell growth and cell adhesion regulatory properties (11).
Here we tested the ability of PTEN to suppress glioma cell growth, whether some naturally occurring mutations ablated this ability, and, whether an intact catalytic phosphatase domain was required for this activity. These results provide functional evidence in support of the candidacy of the PTEN gene as a targeted chromosome 10 suppressor gene in gliomas and confirm that this capacity is effected through its phosphatase activity.
MATERIALS AND METHODS
Cell Lines.
The glioma cell lines used in this study were described previously (16–18). Each was derived from glioblastoma tumors, except LN319 and U373, which originated as anaplastic astrocytomas, and D247, which originated as a gliosarcoma. The normal human fibroblast, MRC-5, was obtained from the American Type Culture Collection.
Vector Construction and Cloning of PTEN Alleles.
A hemagglutinin antigen (HA) shuttle vector was created by PCR amplification of HA from pGETPI by using primers HA3: CCCAAGCTTCATGCCATGGGCCGCATCTTTTACCCATAC and HA4: CCGGAATTCAGCACTGAGCAGCGTAATCTGG and subcloning the product into the HindIII/EcoRI sites of pBluescriptSK− (Stratagene) to generate pBS-HA. To clone PTEN, first-strand cDNA synthesis was accomplished by using adult brain RNA primed with oligo(dT) and random hexamer oligonucleotides and reverse-transcribed with Superscript RNase H− RT (GIBCO/BRL). Subsequent PCR amplification of the PTEN coding region (9) was performed by using primers FF2A: 5′ CCCAAGCTTTGGGATCCGAATTCTACTCCCAGACATGACAGCCATC paired with FF1B: 5′-CATGGAATTCCCATGGCGACTTTTGTAATTTGTGTATGC or paired with FF2B: 5′-CATGGAATTCTCAGACTTTTGTAATTTGTGTATGC. PCR conditions were 55°C for annealing and 72°C for extension for 30 cycles by using Pfu DNA polymerase (Stratagene) and standard conditions. PCR products generated with primer pair FF2A/FF1B were subcloned into HindIII/NcoI sites of pBS-HA, and products generated with primer pair FF2A/FF2B were subcloned into the EcoRI site pBluescriptSK− to generate pBS-PTEN-HA and pBS-PTEN, respectively. All constructs were sequenced entirely to eliminate PCR-generated artifacts. The resulting wild-type PTEN cDNAs either tagged with the HA at the carboxy terminus or untagged were subcloned into the EcoRI site of pBABE-puro (pBP) (19) to generate pBP-PTEN and pBP-PTEN-HA. Site-directed mutagenesis by recombinant PCR was used to construct mutant PTEN alleles with a point mutation (G129R) or two deletions (d55/70, lacking amino acids 55–70, and d237/239, lacking amino acids 237–239). For ribonuclease protection analysis (see below), pBP-PTEN was digested with PstI to generate a 417-bp fragment composed of 300 bp of vector sequence appended to 117 bp of PTEN 5′ sequence and subcloned into pBluescriptSK− to generate pBS-Pst-RPA.
Nucleic Acid Isolation and RNA Analysis.
RNA was isolated by using the Trizol reagent (GIBCO/BRL), and Northern blotting was performed as described (20). RNA quality and blotting efficiency were confirmed by reversible methylene blue staining of the blot (21). Subsequent hybridization for Northern analysis was with a 32P-labeled PTEN probe corresponding to the full-length coding region as described above or, for ribonuclease protection, with 500-bp and 128-bp antisense RNA probes generated from XhoI-digested pBS-PST-RPA and pTRI-RNA-18S (Ambion), respectively. Blots were exposed to PhosphoImager screens for 12 hr, and images were generated with ImageQuant software (Molecular Dynamics).
Protein Truncation Test (PTT) and Sequencing of PTEN cDNAs.
PTT templates were generated by PCR from first-strand cDNA by using primer T7PTEN2: 5′-GGATCCTAATACGACTCACTATAGGAACAGACCACCCAAGTCCAGAGCCATTTCCATCCTGCAG paired with FF2B. The resulting PCR products were purified by QIAquick PCR columns (Qiagen), and 100 ng of product was used in a TNT T7 Quick Coupled Transcription/Translation reaction (Promega) with the addition of 0.3 mM magnesium acetate. PTT products were resolved through 10% discontinuous SDS/PAGE. Gels were fixed, soaked in Amplify (Amersham), dried, and exposed to a PhosphoImager screen for 12 hr. To localize PTEN mutations, PTT templates were sequenced directly by using an Applied Biosystems automated sequencer.
Transfection Assays.
Transfections were performed by using the calcium phosphate transfection method (22) in triplicate dishes and in at least three independent experiments. Briefly, 10 μg of pBP-PTEN, pBP-PTEN-HA, or mutant constructs were used per 10-cm dish containing cells seeded at 7 × 105 cells per dish 24 hr before transfection. Transfections were terminated at 6 hr, and at 24 hr posttransfection cells were split at a 1:3 dilution and maintained for 7 days in puromycin-containing media (U87 and LN229 at 500 ng/ml and U178 at 400 ng/ml) (Calbiochem), containing serum lowered to 2%. Trypan blue excluding cells were counted by using a hemocytometer.
HA Immunostaining.
Cells were transfected on glass coverslips and fixed 48 hr posttransfection in 4% paraformaldehyde/PBS followed by methanol. Immunofluorescence incubations were performed at room temperature. Anti-HA was diluted to 1 μg/ml in blocking buffer (PBS/0.1% saponin/2% BSA) and incubated with cells for 1 hr. Coverslips were washed four times with PBS/0.1% saponin and incubated for 1 hr with fluorescein-conjugated goat anti-mouse secondary antibody (Southern Biotechnology Associates) diluted 1:100 in blocking buffer. After secondary antibody incubation, cells were washed four times in PBS/0.1% saponin and twice in PBS. Nuclei were stained with Hoechst 33258, and then the coverslips were placed into antifade medium, mounted, and viewed with a Zeiss fluorescent microscope. For negative primary antibody controls, cells were transfected with pBABE-puro or pBP-PTEN and treated for immunofluorescence as described.
Western Blot Analysis.
Cells from 10-cm plates 48 hr posttransfection were washed and scraped into cold PBS, pelleted, and lysed with 200 μl RIPA buffer (10 mM Tris⋅HCL, pH 7.5/150 mM NaCl/2 mM EDTA/1% Nonidet P-40/1% sodium deoxycholate/0.1% SDS) containing 1× complete protease inhibitors (Boehringer Mannheim) with the addition of 0.7 μg/ml pepstatin, 1 mM sodium fluoride, 1 mM sodium vanadate, and 0.4 mM microcystin. Thirty micrograms of each lysate was resolved through 10% discontinuous SDS/PAGE and transferred onto poly(vinylidene difluoride) membranes. Membranes were probed with anti-HA antibodies (HA.11, Babco, Richmond, CA), and proteins were detected by chemiluminescence (Amersham).
RESULTS
Expression and Mutation of PTEN in Glioma Cell Lines.
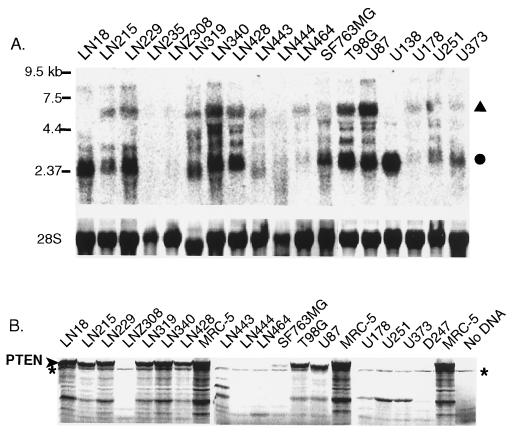
To determine which glioma cell lines would be appropriate recipients to assess the biological effect of ectopic PTEN expression, we first characterized a panel of such lines for their endogenous expression and mutation of the gene. To examine the expression profile of PTEN in glioma cell lines, the cDNA sequence corresponding to the protein coding region of the PTEN mRNA was cloned by reverse transcription–PCR (RT-PCR) from human brain RNA and used as a probe for Northern blot analysis (Fig. 1A). Major transcripts of 5.5 and 2.5 kb and lower abundance transcripts of ≈8, 4.2 and 3 kb were detected in numerous cell lines with the noticeable exception of LN235 and LNZ308, which were devoid of expressed PTEN transcripts. Cell lines such as LN18 and U138 contained a predominant single transcript of 2.5 kb. A previous study has indicated that these various sized PTEN transcripts likely are caused by the use of alternate polyadenylation sites (10).
Figure 1.
PTEN expression and PTT mutation detection in glioma cell lines. (A) Northern blot analysis of PTEN. Major 5.5-kb (▴) and 2.5-kb (•) transcripts are indicated. (Lower) Relative levels of RNA loading are shown as methylene blue staining of 28S RNA. (B) 35S-labeled proteins generated by PTEN PTT. ∗ indicates a nonspecific background product that is present in all lanes. Full-length PTEN is indicated at left.
A PTT was established to rapidly detect PTEN mutations causing stop codons or frameshifts in these cell lines. It has been shown previously that by incorporating a phage promoter into the RT-PCR products and then transcribing and translating in cell free rabbit reticulocyte lysates, frameshift mutations or in-frame deletions can be recognized as the appearance of truncated protein products or the reduction of quantity of full-length protein (23–29). Of the 17 glioma cell lines for which an RT-PCR product could be generated and assayed by PTT, 10 did not yield a full-length protein (Fig. 1B). Among these, cell lines LN443, LN444, D247, and U87 had readily detectable truncations in their RT-PCR products, whereas LN215, LN464, U251, U178, and U373 did not, indicating the likelihood of subtle sequence insertions or deletions in these latter cases. Cell lines such as LN18 were able to express full-length proteins despite expressing a single predominant 2.5-kb transcript (Fig. 1A), whereas LNZ308 cells, which had undetectable expression by Northern blot analysis, yielded a detectable truncated PTEN protein, underscoring the increased sensitivity of the PTT. The frequency of PTEN mutations in glioma cell lines yielding truncated or nonexpressed proteins was 59% (10 of 17) in this first screening assay.
That the truncated proteins were caused by mutation was confirmed by direct sequencing of RT-PCR products. In all cases except for LN215, truncation was caused by sequence deletions causing frameshifts (LNZ308, LN443, LN444, and D247), insertions causing frameshifts (LN464, U178, U251, and U373), or as in the case of U87, an in-frame 15-aa deletion caused by removal of exon 3 (Table 1). LN215 proved to be an exception, with its truncation being caused by a missense mutation (C105F), causing an apparent change in protein mobility. Sequencing of PCR products generated from LN18, LN229, LN428, and SF73MG, which generated full-length PTEN protein, yielded wild-type sequence. However, LN314 and LN344 contained point mutations at the same position, R15I and R15S, respectively (Table 1), a codon also found to be mutated in primary gliomas (10). The combined results from Northern blotting, PTT, and sequencing indicate that 14 of 18 (78%) glioma cell lines contained a mutation of PTEN, a percentage that is somewhat higher than previously reported (59%, ref. 10 and 63%, ref. 9). These mutations are summarized in Table 1. The validity of these results was underscored by our finding that the mutations specific to T98G, U251, U373, and U87 were in agreement with previous reports (9, 10), with U87 being the only in-frame mutant caused by complete removal of an exon.
Table 1.
Summary of PTEN mutations in glioma cell lines
| Cell line | Codon | Mutation | Predicted effect | Detected by |
|---|---|---|---|---|
| LN18 | None | None | PTT/seq | |
| LN215 | 105 | TGT to TTT | Cys to Phe | PTT/seq |
| LN229 | None | None | PTT/seq | |
| LN235 | ND | No expression | Northern/RT-PCR | |
| LNZ308 | Splice variant/del exon 6 | Frameshift | RT-PCR | |
| LN319 | 15 | AGA to ATA | Arg to Ile | Seq |
| LN340 | 15 | AGA to AGT | Arg to Ser | Seq |
| LN428 | None | None | Seq | |
| LN443 | Splice variant/del exon 5 | Frameshift | PTT/seq | |
| LN444 | Splice variant/del exon 5 | Frameshift | PTT/seq | |
| LN464 | 183 | AAG to AAAG | Frameshift | PTT/seq |
| SF763MG | None | None | PTT/seq | |
| T98G | 42 | CTT to CGT | Gly to Gln | Seq* |
| U87 | Splice variant/del exon 3 | In-frame deletion | PTT, seq* | |
| U178 | 248 | CCT to ACCT | Frameshift | PTT/seq |
| U251 | 242 | TTT to TTTT | Frameshift | PTT, seq* |
| U373 | 242 | TTT to TTTT | Frameshift | PTT, seq* |
| D247 | Splice variant/del exon 4 | Frameshift | PTT/seq |
Assessment of the Growth Suppressive Effects of PTEN Alleles in Glioma Cell Lines.
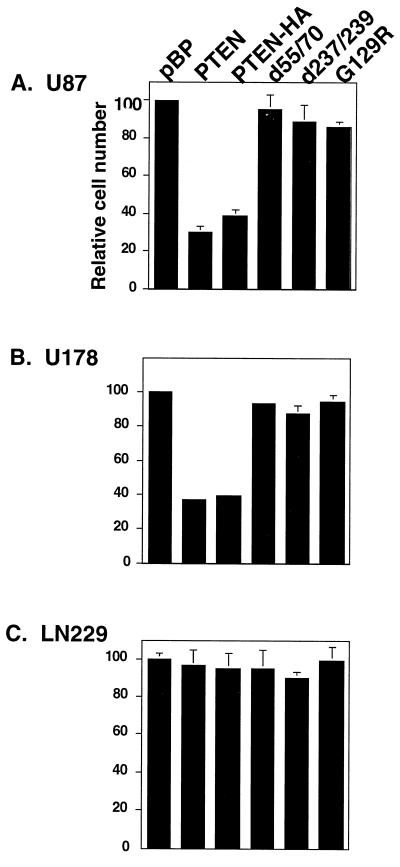
Having determined that mutation or altered expression of PTEN was common among glioma cell lines, we chose U87, U178, and LN229 cells as recipients to examine the effects that introduction of exogenous PTEN had on in vitro growth. U87 contains an in-frame deletion of exon 3 within the tensin region. U178 contains a frameshift in exon 7 downstream of a putative tyrosine phosphorylation site and LN229 is wild type for PTEN expression (Table 1). The wild-type PTEN coding region was cloned into the retroviral expression plasmid, pBABE-puro (19) in forms that were engineered to contain HA sequences at the carboxy terminus or without such a tag. In addition, three mutant alleles of PTEN were constructed: G129R, a mutation within the phosphatase catalytic domain originally detected in a primary glioma tumor (9) and recently shown to lack in vitro phosphatase activity (15); d237/239, a mutation originally described in a primary breast cancer and targeting a putative phosphorylation site (10); and, d55/70, the deletion mutation carried by U87 (10), which targets a conserved α-helix found in protein phosphatases such as PTB1B and VHR (30, 31) and is required for proper secondary structure of the enzyme. To assess the transfection efficiency for each HA-tagged construct, immunostaining for the HA epitope was performed 48 hr posttransfection. By this analysis, 20–23% of U87, 18–20% of U178, and 15–18% of LN229 cells were expressing exogenous PTEN protein. In agreement with a previous report (11), PTEN expression was found mainly in the cytoplasm (Fig. 2). Twenty-four hours after transfection, cells were selected with puromycin for 7 days and subsequently counted. The number of cells in the population transfected with the wild-type PTEN (either with or without the HA tag) was reduced by 60–70% for the two cell lines containing endogenous mutant PTEN alleles, U87 and U178 (Fig. 3) when compared with similar cell populations into which vector was transfected. In contrast, no similar reduction was seen when the experiment was performed with LN229 cells, which contain wild-type endogenous PTEN alleles (Fig. 3). Finally, any of the selected mutations (G129R, d55/70, and d237/239) substantially reduced the growth suppressive effects of PTEN.
Figure 2.
Cellular localization of exogenous PTEN in transfected cells. U87, U178, and LN229 cells were seeded on coverslips and transfected with the indicated plasmids. Expressed HA-epitope-tagged PTEN was detected by immunofluorescence with anti-HA, followed by staining with a fluorescein-conjugated secondary anti-IgG1a antibody. Specificity of the primary antibody is indicated by the lack of staining of untagged PTEN.
Figure 3.
Growth suppressive effect of PTEN alleles in human glioma cell lines. Empty vector (pBP), or vector containing wild-type PTEN, PTEN-HA, or mutant alleles tagged with HA (G129R, d55/70, d237/239) were transfected into (A) U87 and (B) U178 (both mutant PTEN backgrounds), and (C) LN229 (wild-type PTEN background) and then selected with puromycin. Viable cells were counted after 7 days. Results are normalized in terms of percentage of vector transfection with the vector control set to 100% in each case. A typical transfection is shown. Results were reproduced in three independent experiments.
To confirm that the biological effects were not caused by differential transgene expression, RNA and protein cell lysates were prepared from U87, U178, and LN229 cells 2 days after transfection. Ribonuclease protection analyses showed the expected 433-bp fragment representing the introduced PTEN gene at roughly similar levels in all cells except the controls where either no fragment (mock controls) or the 300-bp vector fragment (vector controls) were observed (Fig. 4A). U87 and U178 cells expressed somewhat less exogenous PTEN and PTEN-HA transcripts, whereas PTEN/G129R, d55/70, or d237/239 transcripts were expressed at roughly equal amounts. In contrast, LN229 cells expressed equal amounts of both wild-type and mutant exogenous PTEN transcripts. These results further suggest that cells expressing endogenous mutant PTEN were growth-suppressed upon introduction of exogenous wild-type PTEN and were being rapidly eliminated from the cell population, whereas introduction of mutant alleles lacked this effect. Furthermore, a cell line with a wild-type PTEN background was unaffected by additional PTEN. Protein blots prepared from the same cells and probed with anti-HA antibody showed similar expression of all epitope-tagged proteins (Fig. 4B). An analysis of protein cell extracts performed 7 days post-puromycin selection showed reduced levels of PTEN, which were similar in all cases (data not shown). These results indicate that exogenous PTEN was expressed in transfected cells and conferred growth suppression only when endogenous PTEN was mutated. In contrast, mutants G130R, d55/70, or d237/239, which were similarly expressed, failed to suppress growth.
Figure 4.
Expression of exogenous PTEN alleles in U178, U87, and LN229 cells. (A) Ribonuclease protection analysis of RNA levels from cells transfected with vector, wild-type PTEN, and mutant PTEN alleles. (Upper) 433-bp exogenous protected PTEN fragments (▪) or 300-bp protected vector fragments (•). (Lower) 80-bp protected 18S RNA fragment (▴) from the same gel. (B) (Upper) Western blot analysis of HA-tagged wild-type (▪) and mutant PTEN-HA proteins d55/73 (♦); d237/239 (•), and G129R (▴) 2 days posttransfection. Note that untagged PTEN does not react with the anti-HA antibody. (Lower) Amido black staining of protein blot showing equal loading of protein.
DISCUSSION
Authentication of the candidacy of a suspected tumor suppressor gene requires both a description of its mutation frequency and a demonstration of growth suppressive function in vitro or in vivo. Here we have described the types and frequency of mutations and growth suppression by the recently described putative tumor suppressor gene, PTEN, in glioma cells. A combination of PTT assay and sequence analysis of 18 glioma cell lines showed that 13 of 17 cell lines contained intragenic mutations. In LN235, PTEN was undetectable by Northern and RT-PCR analysis, presumably caused by homozygous genomic deletion (data not shown). The mutation we describe for the U87 cell line would be in agreement with a previous report of a splice donor site mutation in intron 3 (10) causing skipping of exon 3 (32), but is in contrast with another report of a 49-bp deletion inclusive of this exon causing a frameshift (9).
The results of this study, in combination with previous reports (9, 10, 13–15), begin to define preferential regions of mutation in the PTEN gene. These appear to be confined to the tensin/auxilin region at codon 15, the phosphatase catalytic domain within exon 5 (amino acids 86–165), and perhaps codons 232–241, which contain a putative tyrosine phosphorylation site. Consistent with this interpretation, both codon 129 and the putative tyrosine phosphorylation site have been found to be mutated in the germ line of individuals with Cowden disease (12, 13) and Bannayan–Zonana syndrome (14), two autosomal dominant disorders with the hallmark of benign hyperplastic, disorganized growths known as hamartomas. In addition, the G129R mutation has been shown to ablate phosphatase activity (15). The d55/70 mutation, which encompasses Leu-57, an amino acid found mutated in a glioblastoma (L57W) (15) and is important for the proper secondary structure of the protein (30, 31), also would be predicted to ablate phosphatase activity. It will be interesting to test other mutations to determine which inactivate phosphatase activity, which decrease protein stability, and which retain phosphatase activity but perhaps are defective for substrate targeting. In examining the in vitro growth suppressive function of PTEN we selected three glioma cells lines with different PTEN backgrounds: U87 with a deletion of codons 56–70 within the tension/auxilin homology region; U178 with a frameshift at codon 248; and LN229 with a wild-type background. Our data indicate that PTEN conveys growth suppression only in the mutant cell backgrounds. This would suggest that other components in a yet-to-be-described pathway are mutated or altered in cells containing wild-type PTEN, perhaps analogous to mutations of members of the retinoblastoma protein pathway found in nearly all gliomas (33–37). The in vitro growth inhibition caused by PTEN will make in vivo experiments difficult but these are underway using its conditional expression. In any case the present results establish that the PTEN gene is a growth suppressor of glioma cells and the system we have established to test this should prove useful in dissecting its mode of action.
Acknowledgments
We thank N. de Tribolet and E. Van Meir for the LN series of cell lines, M. Anderson and D. Wang for supplying RNA and blots, J. Weger for automated sequencing, B. Amati for pBABE-puro, B. Furnari for pGTEPI, and M. Nagane and G. Robertson for helpful discussions. F.B.F. was supported by the Robert Steel Foundation for Pediatric Cancer Research.
ABBREVIATIONS
- PTT
protein truncation test
- HA
hemagglutinin antigen
- RT-PCR
reverse transcription–PCR
References
- 1.Furnari F B, Huang H J, Cavenee W K. Cancer Surv. 1995;25:233–275. [PubMed] [Google Scholar]
- 2.James C D, Olson J J. Curr Opin Oncol. 1996;8:188–195. doi: 10.1097/00001622-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Louis D N, Gusella J F. Trends Genet. 1995;11:412–415. doi: 10.1016/s0168-9525(00)89125-8. [DOI] [PubMed] [Google Scholar]
- 4.von Deimling A, Louis D N, Wiestler O D. Glia. 1995;15:328–338. doi: 10.1002/glia.440150312. [DOI] [PubMed] [Google Scholar]
- 5.Westermark B, Nister M. Curr Opin Oncol. 1995;7:220–225. doi: 10.1097/00001622-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Rasheed B K, McLendon R E, Friedman H S, Friedman A H, Fuchs H E, Bigner D D, Bigner S H. Oncogene. 1995;10:2243–2246. [PubMed] [Google Scholar]
- 7.James C D, Carlbom E, Dumanski J P, Hansen M, Nordenskjold M, Collins V P, Cavenee W K. Cancer Res. 1988;48:5546–5551. [PubMed] [Google Scholar]
- 8.Bigner S H, Mark J, Burger P C, Mahaley M S, Jr, Bullard D E, Muhlbaier L H, Bigner D D. Cancer Res. 1988;48:405–411. [PubMed] [Google Scholar]
- 9.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 10.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng D H, Tavtigian S V. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 11.Li D M, Sun H. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 12.Nelen M R, van Staveren W C G, Peeters E A J, Ben Hassel M, Gorlin R J, Hamm H, Lindboe C F, Fryns J-P, Sijmons R H, Woods D G, Mariman E C M, Padberg G W, Kremer H. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- 13.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, Eng C, Parsons R. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 14.Marsh D J, Dahia P L M, Zheng Z, Liaw D, Parsons R, Gorlin R J, Eng C. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- 15.Myers M P, Stolarov J P, Eng C, Li J, Wang S I, Wigler M H, Parsons R, Tonks N K. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Meir E, Ceska M, Effenberger F, Walz A, Grouzmann E, Desbaillets I, Frei K, Fontana A, de Tribolet N. Cancer Res. 1992;52:4297–4305. [PubMed] [Google Scholar]
- 17.Van Meir E G, Kikuchi T, Tada M, Li H, Diserens A C, Wojcik B E, Huang H J, Friedmann T, de Tribolet N, Cavenee W K. Cancer Res. 1994;54:649–652. [PubMed] [Google Scholar]
- 18.Carlsson J, Acker H. Int J Radiat Oncol Biol Phys. 1985;11:535–546. doi: 10.1016/0360-3016(85)90185-3. [DOI] [PubMed] [Google Scholar]
- 19.Morgenstern J P, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furnari F B, Adams M D, Pagano J S. J Virol. 1992;66:2837–2845. doi: 10.1128/jvi.66.5.2837-2845.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrin D L, Schmidt G W. Biotechniques. 1988;6:196–200. [PubMed] [Google Scholar]
- 22.Krieger M. In: DNA Transfer. Krieger M, editor. New York: Stockton; 1990. pp. 96–98. [Google Scholar]
- 23.Roest P A, Roberts R G, Sugino S, van Ommen G J, den Dunnen J T. Hum Mol Genet. 1993;2:1719–1721. doi: 10.1093/hmg/2.10.1719. [DOI] [PubMed] [Google Scholar]
- 24.van der Luijt R, Khan P M, Vasen H, van Leeuwen C, Tops C, Roest P, den Dunnen J, Fodde R. Genomics. 1994;20:1–4. doi: 10.1006/geno.1994.1119. [DOI] [PubMed] [Google Scholar]
- 25.Plummer S J, Anton-Culver H, Webster L, Noble B, Liao S, Kennedy A, Belinson J, Casey G. Hum Mol Genet. 1995;4:1989–1991. doi: 10.1093/hmg/4.10.1989. [DOI] [PubMed] [Google Scholar]
- 26.Hogervorst F B, Cornelis R S, Bout M, van Vliet M, Oosterwijk J C, Olmer R, Bakker B, Klijn J G, Vasen H F, Meijers-Heijboer H, Menko F H, Cornelisse C J, den Dunnen J T, Devilee P, van Ommen G-J B. Nat Genet. 1995;10:208–212. doi: 10.1038/ng0695-208. [DOI] [PubMed] [Google Scholar]
- 27.Telatar M, Wang Z, Udar N, Liang T, Bernatowska-Matuszkiewicz E, Lavin M, Shiloh Y, Concannon P, Good R A, Gatti R A. Am J Hum Genet. 1996;59:40–44. [PMC free article] [PubMed] [Google Scholar]
- 28.De Benedetti V M, Radice P, Mondini P, Spatti G, Conti A, Illeni M T, Caligo M A, Cipollini G, Bevilaqua G, Pilotti S, Pierotti M A. Oncogene. 1996;13:1353–1357. [PubMed] [Google Scholar]
- 29.Johannsson O, Ostermeyer E A, Hakansson S, Friedman L S, Johansson U, Sellberg G, Brondum-Nielsen K, Sele V, Olsson H, King M C, Borg A. Am J Hum Genet. 1996;58:441–450. [PMC free article] [PubMed] [Google Scholar]
- 30.Yuvaniyama J, Denu J M, Dixon J E, Saper M A. Science. 1996;272:1328–1331. doi: 10.1126/science.272.5266.1328. [DOI] [PubMed] [Google Scholar]
- 31.Stuckey J A, Schubert H L, Fauman E B, Zhang Z Y, Dixon J E, Saper M A. Nature (London) 1994;370:571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- 32.Krawczak M, Reiss J, Cooper D N. Hum Genet. 1992;90:41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- 33.Petronio J, He J, Fults D, Pedone C, James C D, Allen J R. J Neurosurg. 1996;84:1020–1023. doi: 10.3171/jns.1996.84.6.1020. [DOI] [PubMed] [Google Scholar]
- 34.Ueki K, Ono Y, Henson J W, Efird J T, von Deimling A, Louis D N. Cancer Res. 1996;56:150–153. [PubMed] [Google Scholar]
- 35.Sonoda Y, Yoshimoto T, Sekiya T. Oncogene. 1995;11:2145–2149. [PubMed] [Google Scholar]
- 36.He J, Olson J J, James C D. Cancer Res. 1995;55:4833–4836. [PubMed] [Google Scholar]
- 37.He J, Allen J R, Collins V P, Allalunis-Turner M J, Godbout R, Day R S R, James C D. Cancer Res. 1994;54:5804–5807. [PubMed] [Google Scholar]