Abstract
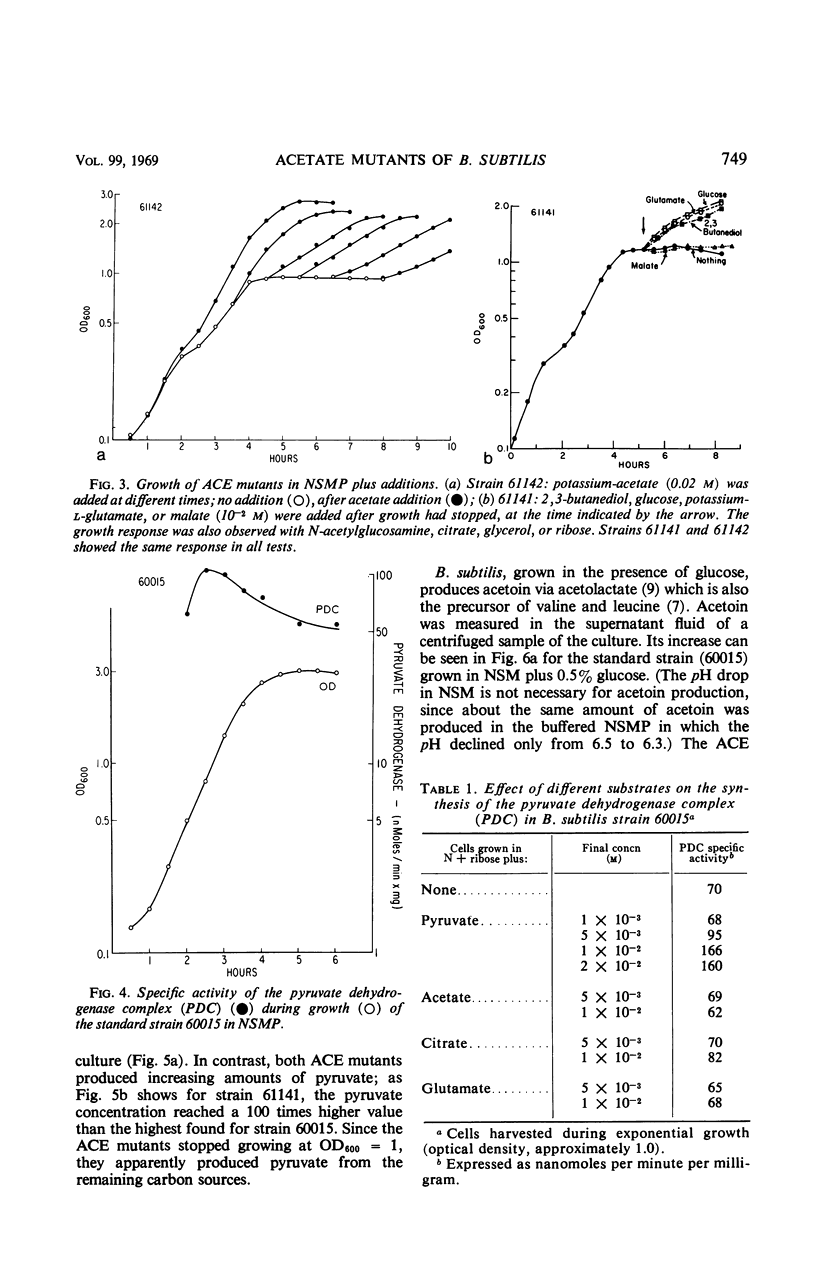
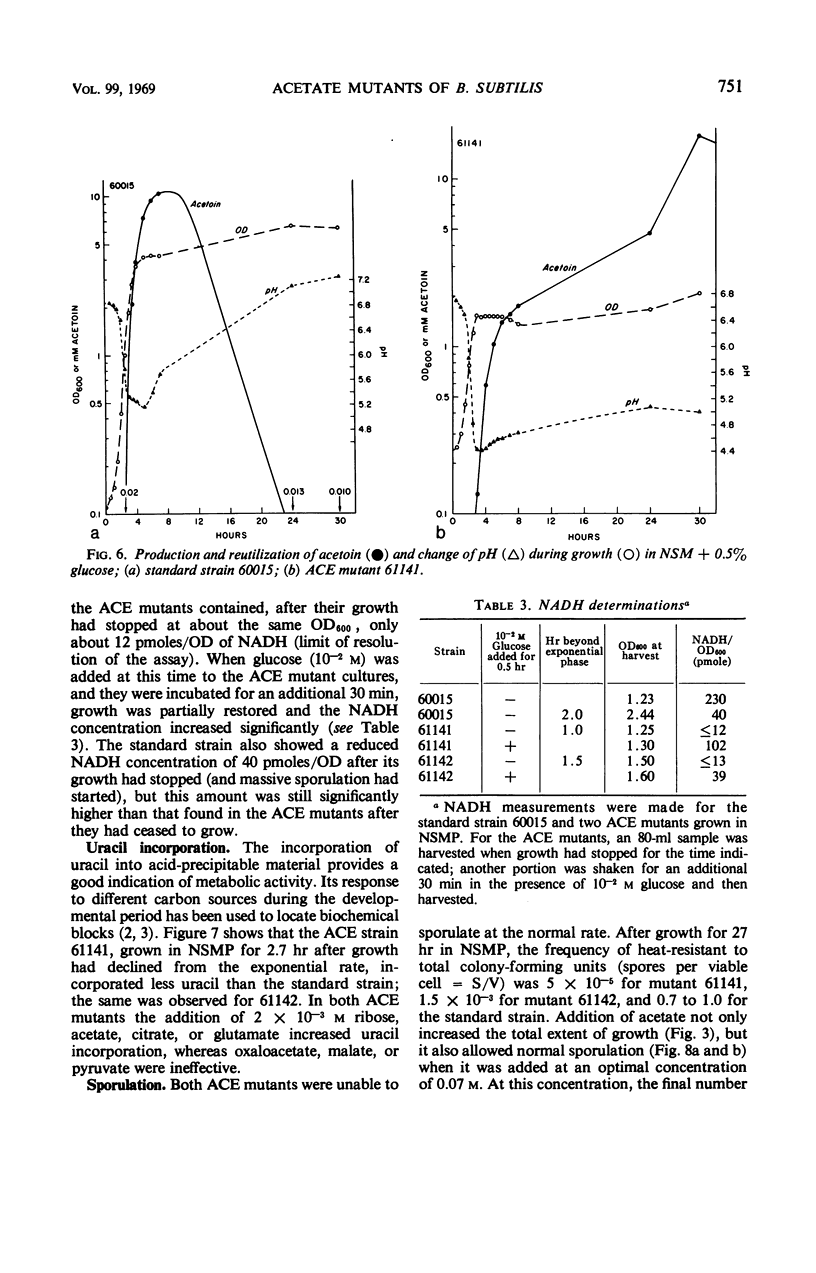
Two “ACE” mutants of Bacillus subtilis which require acetate for growth on glucose minimal medium have been isolated. They do not grow with acetoin, 2,3-butanediol, fatty acids, isoleucine, lipoic acid, malic acid, pyruvic acid, succinic acid, thiamine, or valine, but respond somewhat to glutamate or citrate. The mutants lack the activity of the pyruvate dehydrogenase complex; they excrete pyruvate and later acetoin. They grow in nutrient sporulation medium (NSMP) to one-half the normal turbidity and do not sporulate subsequently. When acetate is added to NSMP (at the optimal concentration of 0.07 m), the ACE mutants grow to the normal turbidity and then sporulate normally. Growth but not sporulation is restored in NSMP upon addition of 2,3-butanediol, citrate, glucose, glutamate, glycerol, or ribose, but not upon addition of acetoin, malate, oxaloacetate, pyruvate, and several other compounds. After growth in NSMP has stopped, the mutants incorporate uracil only at a very low rate, which can be increased by the addition of acetate, citrate, or glutamate. Furthermore, the metabolism of acetoin is prevented after growth has stopped but can be restored by the addition of acetate. All these results can be explained by a lack of reduced nicotinamide adenine dinucleotide (NADH) resulting from the deficiency in acetylcoenzyme A. In fact, after growth of the ACE mutants had stopped, the NADH concentration was at the borderline of measurability, whereas it increased significantly upon addition of glucose. The growing standard strain contains, at the same bacterial turbidity, at least 20 times more NADH (230 pmole/optical density unit at 600 nm) than the nongrowing ACE mutants. The isolated spores, obtained after growth in NSMP plus acetate, can be initiated to germinate in the presence of either l-alanine or the combination of l-asparagine, fructose, glucose, and potassium; addition of acetate is not required and has no effect.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUNARIS A. D., HAGER L. P. A resolution of the Escherichia coli pyruvate dehydrogenase complex. J Biol Chem. 1961 Apr;236:1013–1018. [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Evidence for two distinct enzyme systems forming acetolactate in Aerobacter aerogenes. J Biol Chem. 1959 Dec;234:3067–3071. [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. A cyclic pathway for the bacterial dissimilation of 2, 3-butanediol, acetylmethylcarbinol, and diacetyl. I. General aspects of the 2, 3-butanediol cycle. J Bacteriol. 1956 Apr;71(4):425–432. doi: 10.1128/jb.71.4.425-432.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Mechanisms of formation of acetoin by bacteria. J Biol Chem. 1952 Apr;195(2):715–726. [PubMed] [Google Scholar]
- KORKES S., DEL CAMPILLO A., GUNSALAS I. C., OCHOA S. Enzymatic synthesis of citric acid. IV. Pyruvate as acetyl donor. J Biol Chem. 1951 Dec;193(2):721–735. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- REED L. J., LEACH F. R., KOIKE M. Studies on a lipoic acid-activating system. J Biol Chem. 1958 May;232(1):123–142. [PubMed] [Google Scholar]
- SOKATCH J. T., GUNSALUS I. C. Aldonic acid metabolism. I. Pathway of carbon in an inducible gluconate fermentation by Streptococcus faecalis. J Bacteriol. 1957 Apr;73(4):452–460. doi: 10.1128/jb.73.4.452-460.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R., Freese E. Curing of a sporulation mutant and antibiotic activity of Bacillus subtilis. J Bacteriol. 1968 Oct;96(4):1255–1265. doi: 10.1128/jb.96.4.1255-1265.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Freese E. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of L-alanine. J Bacteriol. 1968 Feb;95(2):433–438. doi: 10.1128/jb.95.2.433-438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



