Abstract
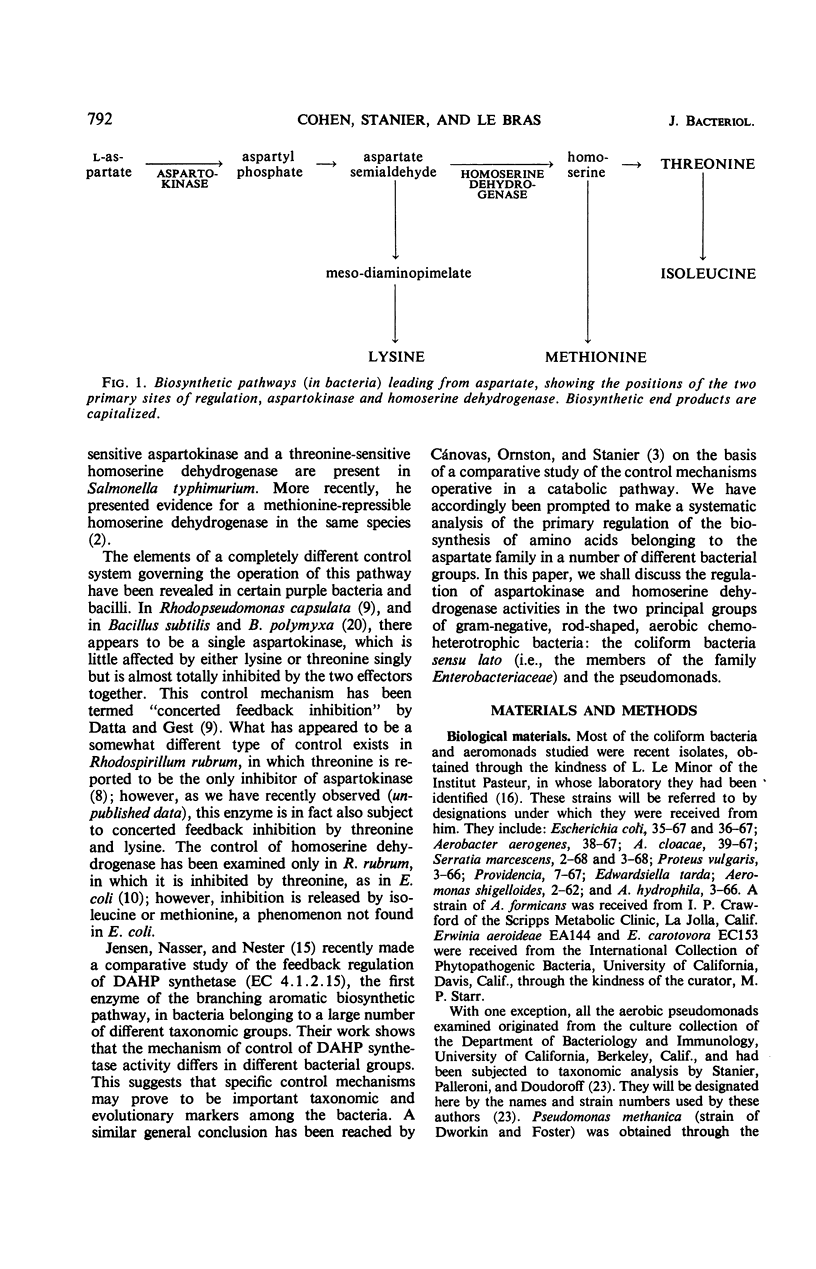
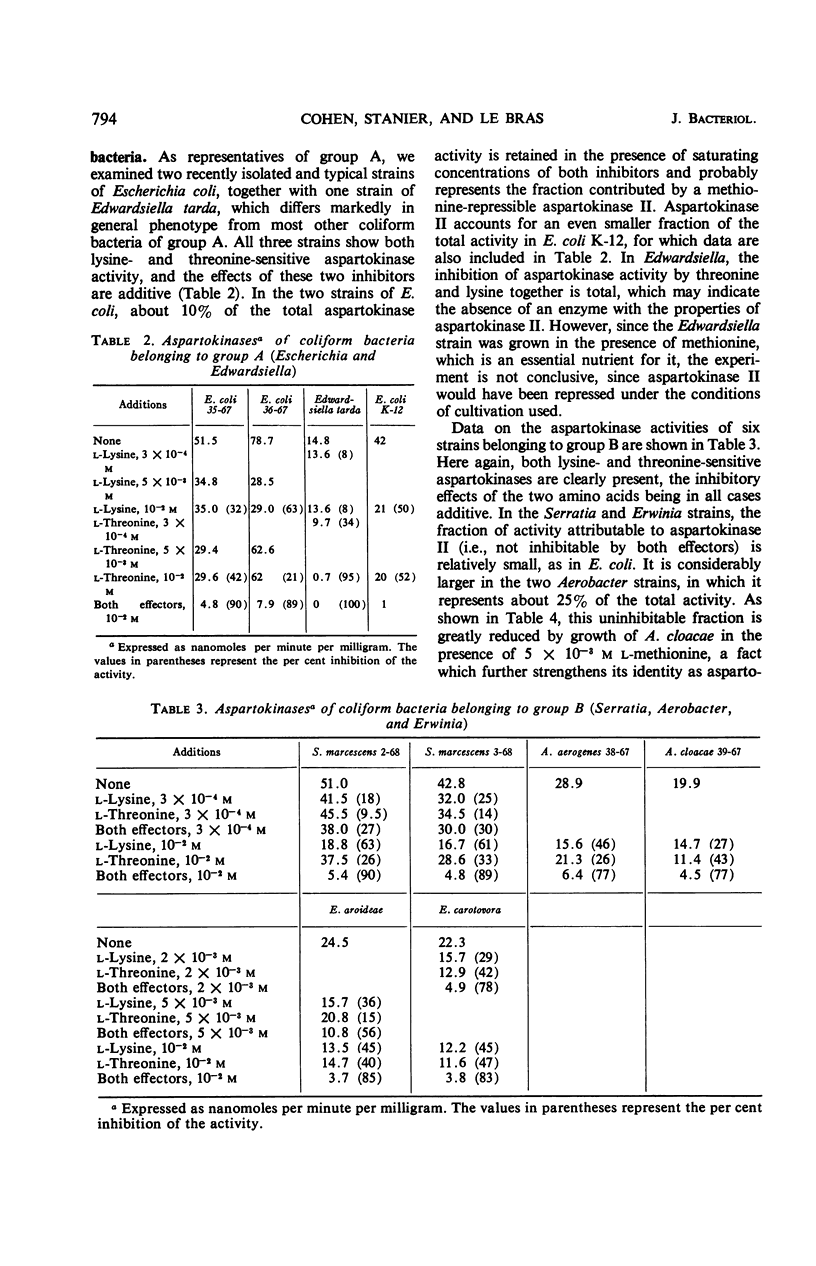
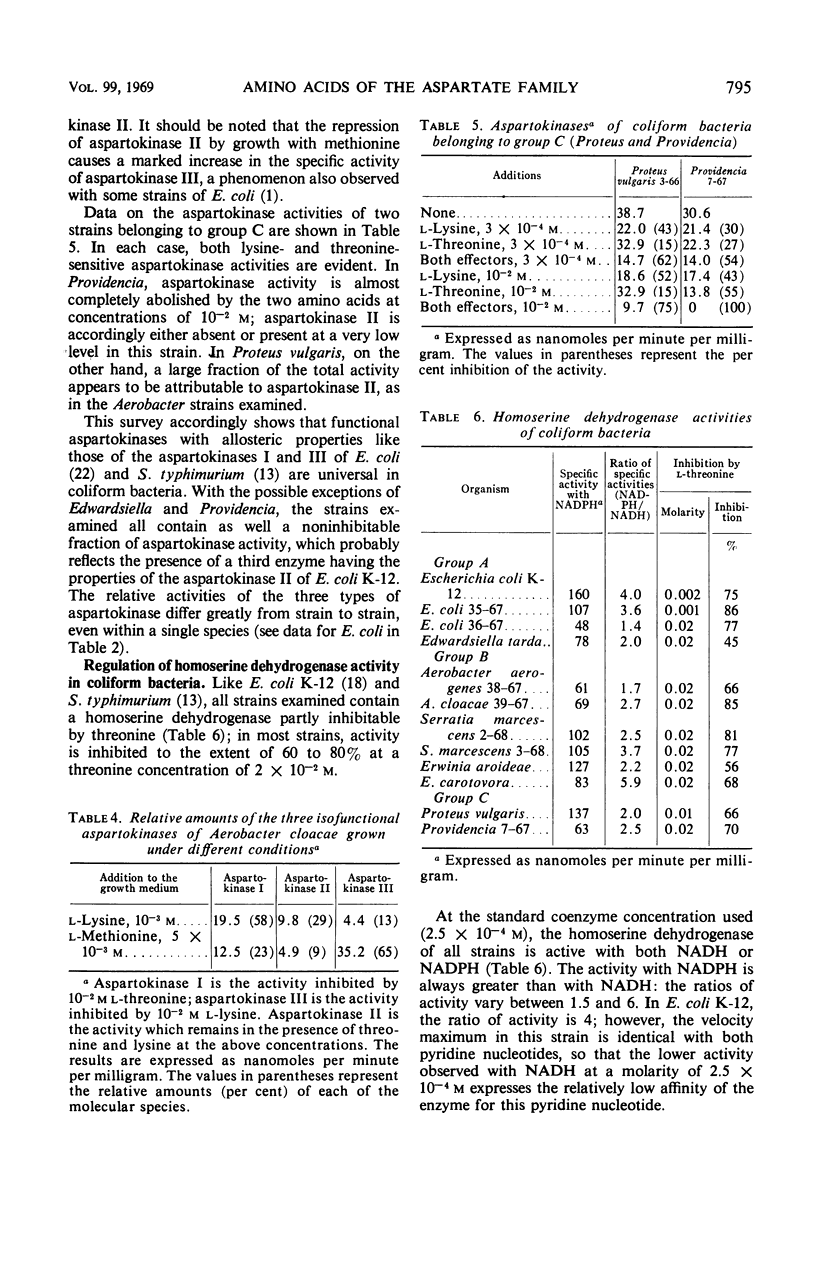
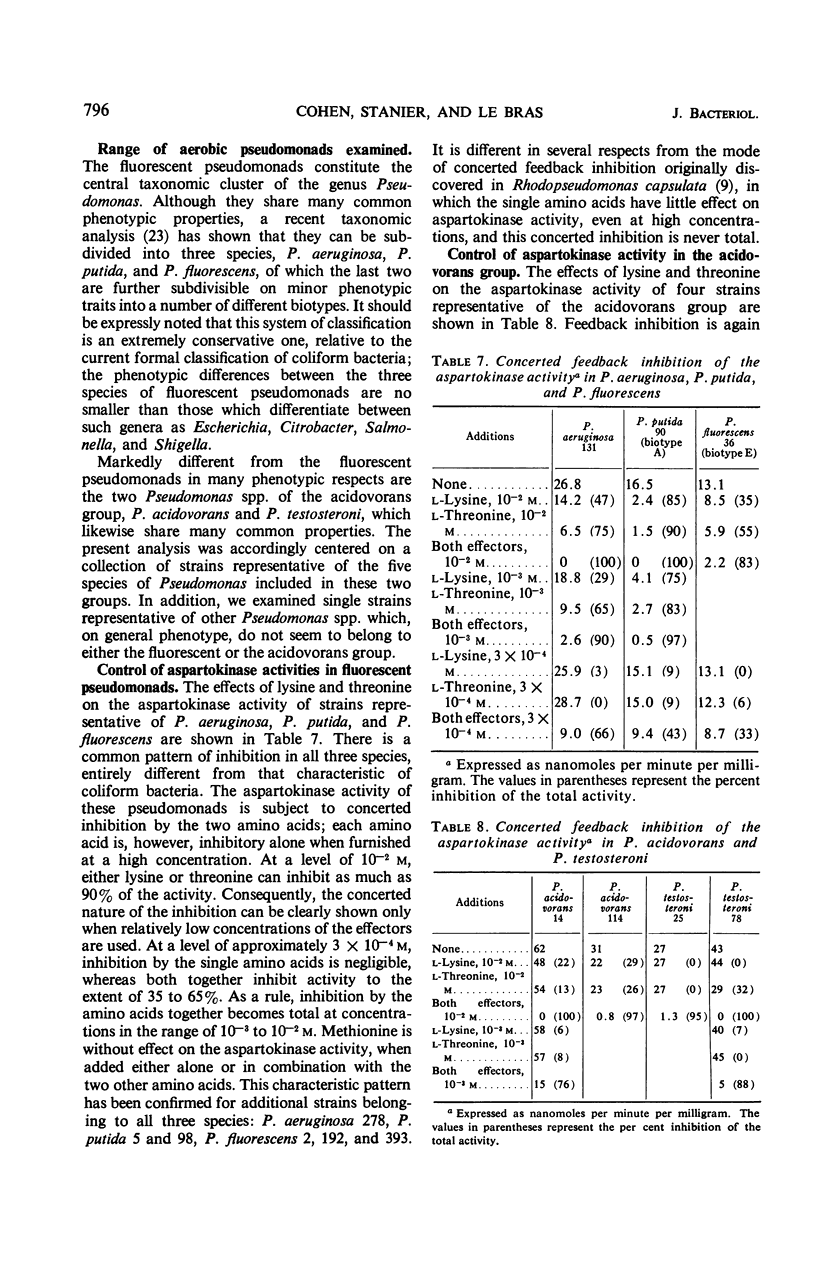
The control of aspartokinase and homoserine dehydrogenase activities was compared in aerobic and fermentative pseudomonads (genera Pseudomonas and Aeromonas), and in coliform bacteria representative of the principal genera of the Enterobacteriaceae. Isofunctional aspartokinases subject to independent end-product control occur in the Enterobacteriaceae and in Aeromonas. In Pseudomonas, there appears to be a single aspartokinase, subject to concerted feedback inhibition by lysine and threonine. Within this genus, the sensitivity of aspartokinase to the single allosteric inhibitors varies considerably: the aspartokinase of the acidovorans group is little affected by the single inhibitors, whereas that of the fluorescent group is severely inhibited by either amino acid at high concentration. In all bacteria examined, homoserine dehydrogenase activity is inhibited by threonine; inhibition is more severe in aerobic pseudomonads than in the other groups. In most of the bacteria examined, either nicotinamide adenine dinucleotide (NAD) or nicotinamide adenine dinucleotide phosphate can serve as a cofactor for this enzyme, though the relative activity with the two pyridine nucleotides varies widely. Aerobic pseudomonads of the acidovorans group contain a homoserine dehydrogenase that is absolutely specific for NAD. The taxonomic implications of these findings are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biswas D. K., Mazumder R., Biswas C. Regulation of aspartate kinase by methionine, threonine, and lysine in Escherichia coli strain B. J Biol Chem. 1968 Jul 10;243(13):3655–3660. [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for a methionine-controlled homoserine dehydrogenase in Salmonella typhimurium. J Bacteriol. 1969 Jan;97(1):193–198. doi: 10.1128/jb.97.1.193-198.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Sikes S., Melhorn D. K. The natural relationships of Aeromonas formicans. Arch Mikrobiol. 1967;59(1):72–81. doi: 10.1007/BF00406318. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- DATTA P., GEST H. ALTERNATIVE PATTERNS OF END-PRODUCT CONTROL IN BIOSYNTHESIS OF AMINO-ACIDS OF THE ASPARTIC FAMILY. Nature. 1964 Sep 19;203:1259–1261. doi: 10.1038/2031259a0. [DOI] [PubMed] [Google Scholar]
- DATTA P., GEST H. CONTROL OF ENZYME ACTIVITY BY CONCERTED FEEDBACK INHIBITION. Proc Natl Acad Sci U S A. 1964 Oct;52:1004–1009. doi: 10.1073/pnas.52.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P., GEST H., SEGAL H. L. EFFECTS OF FEEDBACK MODIFIERS ON THE STATE OF AGGREGATION OF HOMOSERINE DEHYDROGENASE OF RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1964 Jan;51:125–130. doi: 10.1073/pnas.51.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem Biophys Res Commun. 1963 Feb 6;10:277–282. doi: 10.1016/0006-291x(63)90430-3. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., van Rapenbusch R., Cohen G. N. The methionine-repressible homoserine dehydrogenase and aspartokinase activities of Escherichia coli K 12. Preparation of the homogeneous protein catalyzing the two activities. Molecular weight of the native enzyme and of its subunits. Eur J Biochem. 1969 Mar;8(1):146–152. doi: 10.1111/j.1432-1033.1969.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTE J. C., LE BRAS G., LOVINY T., COHEN G. N. [Retro-inhibition and repression of the homoserine dehydrogenase of Escherichia coli]. Biochim Biophys Acta. 1963 Jan 8;67:16–30. doi: 10.1016/0006-3002(63)91793-1. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Rohlfing S. R., Crawford I. P. Purification and characterization of the beta-galactosidase of Aeromonas formicans. J Bacteriol. 1966 Mar;91(3):1085–1097. doi: 10.1128/jb.91.3.1085-1097.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Truffa-Bachi P., Cohen G. N. La beta-aspartokinase sensible à la lysine d'Escherichia coli; purification et propriétés. Biochim Biophys Acta. 1966 Mar 7;113(3):531–541. [PubMed] [Google Scholar]
- Truffa-Bachi P., Van Rapenbusch R., Janin J., Gros C., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K 12. 4. Isolation, molecular weight, amino acid analysis and behaviour of the sulfhydryl groups of the protein catalyzing the two activities. Eur J Biochem. 1968 Jun;5(1):73–80. doi: 10.1111/j.1432-1033.1968.tb00339.x. [DOI] [PubMed] [Google Scholar]



