Abstract
Manganese (Mn) is a transition metal that is essential for normal cell growth and development, but is toxic at high concentrations. While Mn deficiency is uncommon in humans, Mn toxicity is known to be readily prevalent due to occupational overexposure in miners, smelters and possibly welders. Excessive exposure to Mn can cause Parkinson's disease-like syndrome; patients typically exhibit extrapyramidal symptoms that include tremor, rigidity and hypokinesia (Calne et al., 1994; Dobson et al., 2004).
Mn-induced motor neuron diseases have been the subjects of numerous studies; however, this review is not intended to discuss its neurotoxic potential or its role in the etiology of motor neuron disorders. Rather, it will focus on Mn uptake and transport via the orthologues of the divalent metal transporter (DMT1) and its possible implications to Mn toxicity in various categories of eukaryotic systems, such as in vitro cell lines, in vivo rodents, the fruitfly, Drosophila melanogaster, the honeybee, Apis mellifera L., the nematode, Caenorhabditis elegans, and the baker's yeast, Saccharomyces cerevisiae.
Keywords: DMT1, Manganese, NRAMP-2, Transport
Manganese
Mn is one of the most abundant naturally occurring elements in the earth's crust; it does not occur naturally in a pure state. Oxides, carbonates and silicates are the most important Mn-containing minerals (Post, 1999). Depending on its oxidation state, Mn is utilized in countless industrial processes, such as the production of dry cell batteries, steel (Post, 1999; Saric, 1986), fuel oil additives and antiknock agents (Pfeifer et al., 2004; Ressler et al., 1999; Rollin et al., 2005).
The major source of Mn in humans is through dietary ingestion. Approximately 3-5% of ingested Mn is absorbed across the intestinal wall, and the remainder is excreted in feces, representing tight homeostatic control over Mn absorption. Mn toxicity from dietary intake is rare; its uptake is tightly regulated, and any excess of ingested Mn is readily excreted via the bile. In contrast, both pulmonary uptake and particulate transport via the olfactory bulb (Saric, 1986; Aschner et al., 1991; Thompson et al., 2007) can lead to deposition of Mn within the striatum and cerebellum and inflammation of the nasal epithelium (Dorman et al., 2004).
Mn plays an important role in the development and functioning of the brain (Prohaska, 1987; Takeda, 2003). Mn deficiency may result in birth defects, poor bone formation and an increased susceptibility to seizures (Aschner, 2000; Aschner et al., 2002). As an essential metal, it is a co-factor for many enzymes such as transferases, hydrolases, lyases, glutamine synthetase and integrins (Saric, 1986; Wedler et al., 1984). The most well studied Mn-containing polypeptides are arginase, an enzyme present in lipids that is required for ammonia elimination, and Mn-containing superoxide dismutase (Mn-SOD), a principal anti-oxidant enzyme typically found in the mitochondria. Moreover, Mn plays a role in the modulation of the immune system, and in protein, lipid and carbohydrate metabolism (Addess et al., 1997; Aschner et al., 1992; Fitsanakis et al., 2005; Malecki et al., 1999). Evidence also corroborates the involvement of Mn in the stellate process production in astrocytes (Liao et al., 2001), as well as in the metabolism of brain glutamate to glutamine, a step carried out by the astrocyte-specific enzyme, glutamine synthetase (Wedler et al., 1982; Wedler et al., 1984; Takeda, 2003).
Despite its essentiality in multiple metabolic functions, Mn can be toxic at high concentrations. The brain, in particular, is highly susceptible to Mn toxicity. Occupational exposure to Mn for periods from 6 months to 2 years can cause extrapyramidal symptoms that resemble idiopathic Parkinson's Disease, a disease known as manganism. Patients suffering from manganism exhibit a signature biphasic mode of physical decline, which comprises of an initial phase of psychiatric disturbance and neurological deficits which are followed by motor defects such as akinetic rigidity, dystonia and bradyskinesia (Calne et al., 1994; Olanow, 2004).
Mn Transport
Due to the delicate relationship between Mn's essentiality and toxicity, Mn homeostasis is vital for the optimal functioning of any organism. Although some research has focused on mechanisms associated with the transport of Mn across the blood-brain barrier (BBB), the exact identity of the carrier(s) involved in Mn trafficking into the brain is still controversial. During the past two decades, various transport mechanisms have been identified, including active transport (Murphy et al., 1991) and facilitated diffusion (Rabin et al., 1993; Aschner et al., 1994). More recently, it has been established that Mn can also be transported via high affinity metal transporters such as calcium (Ca) and iron (Fe) transporters. Some of these transporters include the divalent metal transporter (DMT1), which belongs to the family of natural resistance-associated macrophage protein (NRAMP) (Gunshin et al., 1997; Garrick et al., 2003); ZIP-8, a member of the solute carrier-39 (He et al., 2006); transferrin (Tf) receptor (TfR) (Davidsson et al., 1989; Aschner et al., 1994), which is known to be responsible for Fe3+ uptake; voltage regulated (Lucaciu et al., 1997) and store-operated Ca2+ channels (Riccio et al., 2002); and the ionotropic glutatmate receptor Ca2+ channels (Kannurpatti et al., 2000) (Figure 1). While the relative contribution of each of these transporters remains unknown, it is likely that optimal tissue Mn concentrations are maintained by the involvement of all of these transporters. This review will focus on the role of DMT1 in the transport of Mn in several eukaryote systems.
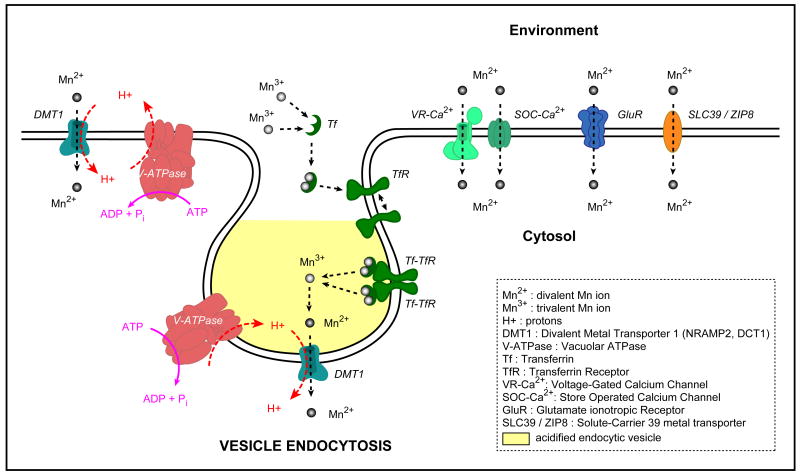
Figure 1. Molecular mechanisms of Mn uptake across the membrane at the blood-brain barrier (BBB).
DMT1 is a symporter energized by the proton-motive force generated by the Vacuolar-ATPase (V-ATPase) which extrudes protons from the cell. The uptake of protons from the extracellular space provides the energy for the transport of Mn2+ cations into the cell. The V-ATPase-generated proton gradient is also responsible for the acidification of endocytic vesicles. Upon acidification, Mn3+ ions released by the transferrin-transferrin receptor system are converted to Mn2+ ions available for transport by DMT1. Transporters such as the voltage-gated and store-operated calcium channels, the glutamate ionotropic receptor and the solute carrier 39 family member ZIP8, are suggested to play a role in Mn uptake at the BBB.
Divalent metal transporter-1
DMT1, previously known as NRAMP2, was first identified in 1995 in a screening for homologues of NRAMP1, a protein involved in host defense against several types of infection (Gruenheid et al., 1995; Vidal et al., 1995). Subsequently, it was referred to as divalent cation transporter (DCT1) because of its ability to transport cations, such as zinc (Zn2+), Mn2+, cobalt (Co2+), cadmium (Cd2+), copper (Cu2+), nickel (Ni2+), lead (Pb2+), and Fe2+ (Forbes et al., 2003; Gunshin et al., 1997; Knopfel et al., 2005). Soon after, Fleming et al. discovered that a missense mutation in the DCT1 in rats or mice lead to deficiency in Fe uptake, confirming that DCT1's major function is cation transportation. In 1999, the nomenclature changed and the transporter was designated as DMT1 (Andrews, 1999).
DMT1 represents a large family of metal ion transporters that are highly conserved from bacteria to humans (Cellier et al., 1995). DMT1 orthologues are highly hydrophobic integral membrane proteins containing 11 to 12 transmembrane domains (TMD). Both the amino- and carboxy- termini are predicted to reside within the cytoplasm (Gruenheid et al., 1995). Members of the DMT1 family share a conserved “consensus transport sequence” which is involved in divalent metal ion translocation, as depicted in the sequence alignment in Figure 2.
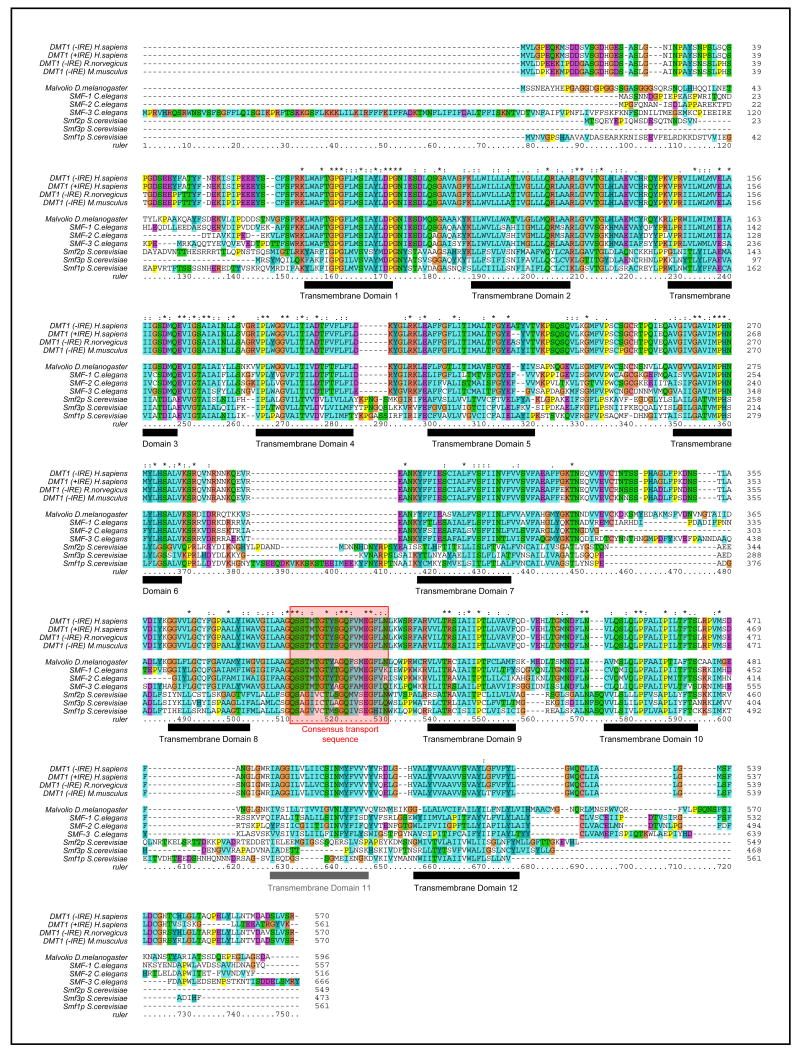
Figure 2. Multiple alignments of DMT1 orthologues in various model organisms.
Two splice variants of DMT1 (-IRE and +IRE) are expressed in vertebrates (H. sapiens, R. norvegicus, M. musculus) differing for the very last 18 or 25 amino acids. Only the −IRE is reported here for the rat and the mouse isoforms. DMT1 orthologues are also encoded by the genomes of the fly, D. melanogaster (Malvolio), the nematode, C. elegans (SMF-1, -2, -3) and the baker's yeast, S. cerevisiae (Smf1p, 2p, 3p). All orthologues share the same topology with 12 conserved transmembrane domains (TMD, black and gray boxes) except for the yeast variants, which only contain 11 predicted TMD. All of them also contain a consensus transport sequence between TMD8 and TMD9 (red box).
The human DMT1 gene consists of 17 exons, spanning more than 36 kb and encoding two proteins of 561 and 570 amino acids. The two alternatively spliced mRNA differ by a specific sequence either containing or lacking an iron regulatory element (+IRE and −IRE respectively). They encode two transporters with distinct carboxy- termini (Fleming et al., 1998; Lee et al., 1998). The 3′ untranslated region (UTR) that contains an IRE has a stem-loop structure whose stability is modulated by the intracellular Fe pool (Gunshin et al., 1997). In the −IRE form, 18 amino acids of the C terminus are replaced by a 25 amino acid segment predicted to be required for an IRE-independent mode of metal regulation (Fleming et al., 1998). It is known that an IRE is also present in the 3′-UTR of the TfR that mediates the transport of Fe (Gunshin et al., 1997). This similarity suggests that DMT1 protein levels may be controlled by intracellular Fe concentration.
DMT1 Localization
Within a cell, DMT1 is mainly localized at the membrane level. Although the two isoforms of DMT1 produced by alternative splicing are both present at the plasma membrane, the difference in the subcellular localization appears to be dependent upon cation needs. The +IRE isoform is found mainly at the apical membrane of epithelial cells (Canonne-Hergaux et al., 1999; Courville et al., 2006) and late endosomes and lysosomes within HEp-2 cells (Tabuchi et al., 2000; Lam-Yuk-Tseung et al., 2005b). On the other hand, -IRE isoforms are found predominantly in early and recycling endosomes (Gruenheid et al., 1999; Kannurpatti et al., 2000; Touret et al., 2003). In addition, DMT1 also has been shown to colocalize with TfR, both at the plasma membrane and in the recycling endosome (Gruenheid et al., 1999).
At the tissue level, DMT1 is ubiquitously expressed, most notably in the proximal duodenum, as compared to areas such as the kidney or the brain (Andrews, 1999; Gunshin et al., 1997; Lee et al., 1998). Immunostaining studies suggest that DMT1 expression is strongest at the brush border of the apical pole of the enterocytes, which is consistent with the role for DMT1 in luminal metal uptake (Canonne-Hergaux et al., 1999). Moreover, recent immunohistochemical experiments revealed that DMT1 is also expressed in the lumen microvilli and end-feet of the sustentacular cells of the olfactory epithelium, suggesting an alternative route of metal exposure (Thompson et al., 2007). In macrophages, DMT1 is restricted in the phagosomal membrane where red cells are engulfed. Using light and electron microscopy, Wang et al. showed extensive DMT1 localization within glial cell bodies of the neocortex, the subcortical white matter and the hippocampus of monkeys (Wang et al., 2001). In the basal ganglia, immunocytochemical experiments indicate dense DMT1 staining in the caudate nucleus, the putamen and the substantia nigra pars reticulata (Huang et al., 2004). This suggests a higher expression of DMT1 within certain areas of the basal ganglia, which may explain the sensitivity of this region to Mn toxicity and the overlap in motor deficits observed in patients suffering from PD and manganism. In rats, DMT1 expression is highest in astrocytes and endothelial cells within the striatum, granule and Purkinje cells of the cerebellum, in neurons within the thalamus and in ependymal cells lining the third ventricle (Burdo et al., 2001; Tandy et al., 2000), with moderate staining in the substantia nigra (Gunshin et al., 1997).
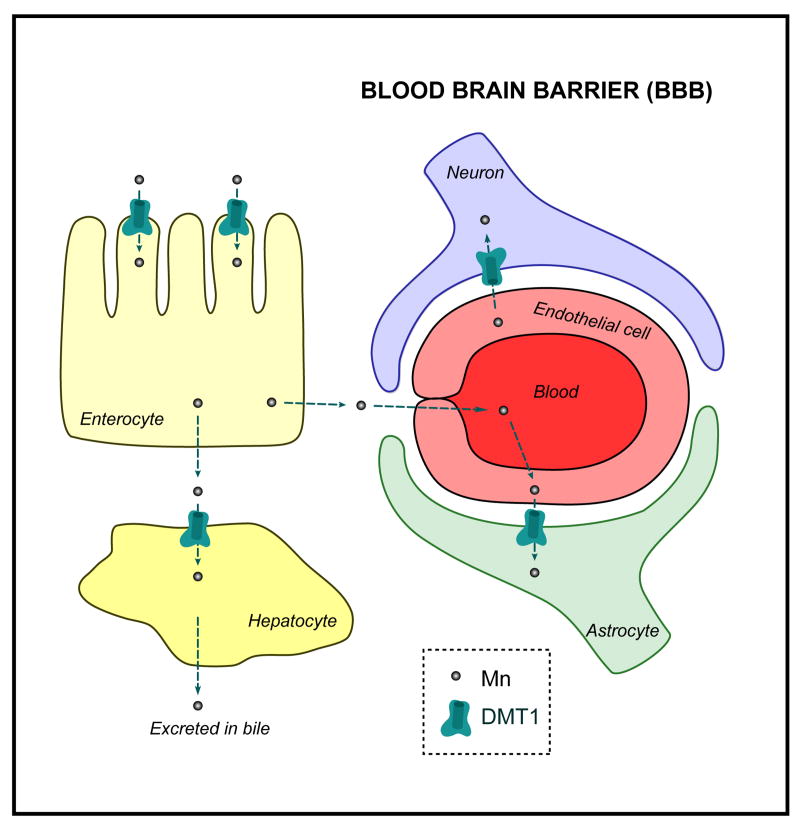
Based on the existing knowledge on the localization of DMT1, the proposed pathway for Mn uptake by the gastrointestinal tract and its transport into the blood stream and across the BBB is depicted in Figure 3.
Figure 3. Mn uptake via DMT1 in vertebrates.
DMT1 is present at the plasma membrane where it is responsible for Mn cellular uptake. Mn is primarily taken up by entorocytes via DMT1 before reaching the adjacent tissues and the blood. Part of it is then excreted in the bile after absorption by the liver, while some accumulates in the brain after crossing the BBB via DMT1 expressed in glial cells and neurons. Mn uptake mechanisms independent of DMT1 are not represented here.
Function of DMT1
DMT1 is important in maintaining the stable homeostasis of essential elements in the brain's extracellular fluids for optimal brain function. The biological function of DMT1 was first determined through phenotypic analysis of two types of animal species that display a spontaneous mutation in the transporter: microcytic (mk) mice and Belgrade (b/b) rats. These animals have naturally occurring mutations that result in substitutions from glycine to arginine at residue 185 (G185R) in the TMD 4 of the DMT1 gene (Su et al., 1998). Both animal species exhibit severe microcytic anemia that is associated with an impairment of Fe transport as well as an alteration in Mn homeostasis. Similarly, humans with DMT1 mutations at different intron or exon sites, such as an E399D substitution (Mims et al., 2005; Lam-Yuk-Tseung et al., 2005a; Priwitzerova et al., 2005), a R416C substitution (Iolascon et al., 2006; Lam-Yuk-Tseung et al., 2006) or a G212V substitution (Beaumont et al., 2006) exhibited hypochromic microcytic anemia, proposing a possible commonality in DMT1 function amongst various mammalian species.
Currently, there are two theories that explain the mechanism of DMT1 functioning: 1) a TfR independent pathway and 2) a TfR dependent pathway. In the TfR independent pathway, it is hypothesized that DMT1 can act as a symporter that couples the pumping of a proton when metal ions are taken up (Garrick et al., 2003; Gunshin et al., 1997). Electrophyiological studies in Xenopus oocytes suggest that at neutral membrane potential and pH, DMT1 is in a 1:1 proton: metal ion transport state (Sacher et al., 2001). An increase in the driving force for metal ion uptake can be achieved by a reduction in pH or a decrease in membrane potential known as the metal-ion dependent proton slip. This tight regulation protects cells against environments containing excessive metals or acidic conditions (Nevo, 2007). Alternatively, the co-localization of DMT1 and TfR suggests that there exists an Mn uptake pathway by DMT1 that is regulated by TfR. When a metal, such as Fe or Mn, binds to the Tf-TfR complex, it causes internalization of the complex from the plasma membrane into the cellular endosomes. V-ATPase is then being recruited and causes the metal-containing endosome to acidify and the metal to dissociate. This will, in turn, activate the DMT1 that is situated at the endosomal membrane to co-transport the metal ion along with a proton into the cytosol as shown in Figure 1 (Gruenheid et al., 1999).
Manganese and DMT1
As mentioned above, the transport of cations via DMT1 is not metal specific. The most direct measure on whether DMT1 plays a role in Mn transport is by the evaluation of changes in DMT1 expression in the presence of excess Mn. An in vivo study by Garcia et al. showed that DMT1 expression increases by ∼35% in the brains of rat pups nurtured by dams fed with high Mn diet. This elevation in DMT1 expression is not region-specific; nonetheless, the data directly relate the effect of an enhanced Mn diet with augmentation in DMT1 expression (Garcia et al., 2006). In addition, this correlation is supported by in vitro data where exposure to Mn for 24 and 48 hours has been shown to increase DMT1 expression in the immortalized choroidal epithelial Z310 cell line by 45% and 78% respectively (Wang et al., 2006).
DMT1 transport of Mn in Saccharomyces cerevisiae
The understanding of the network involved in the Mn uptake mechanism has also greatly benefited from molecular studies of the simple eukaryotic organism, the baker's yeast, Saccharomyces cerevisiae (S. cerevisiae). There are three known DMT1 orthologues expressed in yeast cells: Smf1p, Smf2p and Smf3p, which are encoded by SMF1, SMF2 and SMF3 respectively. Overall, the three Smf proteins exhibit ∼26% identity to human NRAMP2 and ∼48% identity to each other at the amino acid level (Cellier et al., 1995; Portnoy et al., 2000; West et al., 1992). In contrast to vertebrate DMT1, no typical IRE is found in any of the SMF genes, and the poor sequence conservation of the C-termini is not predictive of a possible correspondence between the vertebrate −IRE/+IRE and the Smf1p/2p/3p. These three yeast orthologues are predicted to contain 11 TMDs instead of 12. However, they exhibit a well conserved “consensus transport sequence” which is a signature for the DMT1 family (Figure 2). This sequence conservation implies a functional similarity between human DMT1 and yeast Smfps. Genetic screens first identified the Smf1p transporting Mn across the plasma membrane of yeast cells (Supek et al., 1996). Subsequently, Smf1p was found to be a non-specific metal ion transporter capable of delivering a broader spectrum of essential metal ions such as Mn2+, Zn2+, Cu2+, Fe2+ and Cd2+ (Eide, 1998; Nelson, 1999; Supek et al., 1996). Later, it was shown that the yeast genome encodes two additional orthologous proteins, Smf2p and Smf3p, which might also be involved in metal ion transport, but display distinct substrate specificity from Smf1p, implying partially distinct functions for the yeast Smf proteins. This was confirmed in a study by Nelson et al. in 2000, which showed that only cells overexpressing Smf1p or Smf2p, but not Smf3p, in the triple null mutants lacking SMF1, SMF2 and SMF3 can restore the uptake activity of Mn comparable to the wild type level (Cohen et al., 2000).
The specificity in function of the three Smf proteins may be related to the difference in the subcellular localization of the three proteins. In animals, the transporter can be located both at the cell surface and in intracellular organelles (Nelson, 1999), whereas in S. cerevisiae, the different isoforms are situated at distinct cellular sites: Smf1p at the cell surface, Smf2p in the intracellular vesicles and Smf3p at the vacuolar membrane (Portnoy et al., 2002; Portnoy et al., 2000; Supek et al., 1996).
Although these three proteins share a high resemblance in amino acid sequence and topology, their roles in metal homeostasis are unique. Under physiological conditions, i.e. when cells have a sufficient amount of Mn2+ ions, Smf1p appears to contribute little to cellular Mn levels. This was corroborated in mutants that lack SMF1 where Mn accumulation was indistinguishable from wild type controls (Supek et al., 1996). Yeasts containing a deletion of the SMF2 gene lose the ability to acquire normal levels of the metal (Luk et al., 2001; Pinner et al., 1997). SMF3 mutants do not show any impairment in the uptake and homeostasis of Mn; rather, Smfp3 is mainly responsible for trafficking Fe. The SMF3 promoter contains a recognition sequence for the Fe-sensing transcription factor Aft1p which is responsible for its transcriptional regulation by Fe, whereas Smf1p and Smf2p levels are unaffected by Fe concentration (Portnoy et al., 2002; Portnoy et al., 2000). Since no significant staining of Smf2p or Smf3p was observed at the plasma membrane, and SMF1 mutants do not show changes compared to wild type, it has been hypothesized that some extracellular metal transporter(s) other than the three Smfs is responsible for Mn uptake under physiological conditions.
However, the localization of Smf1p and Smf2p, but not Smf3p, can change, depending upon the surrounding environment. A study by Liu et al. demonstrated that when yeast cells are supplied with excess amounts of Mn, the bulk of Smf1p transporter is targeted to the vacuole for degradation to inhibit further uptake of the metal ion, whereas, in conditions of Mn deficiency, Smf1p undergoes a conformational change that prevents it from entering the vacuole, and the transporter is targeted to the plasma membrane to facilitate the uptake of Mn (Jensen et al., 2003; Liu et al., 1999a, b). This relocation phenomenon also holds true in the case of Smf2p. Under metal replete conditions, there is an intense staining pattern within the lumen of the vacuole, since the majority of the protein is being degraded by vacuolar proteases. In the Mn depleted state, Smf2p is restricted to intracellular punctuate bodies for mobilizing any Mn that is delivered by Smf1p from the extracellular space into the cytosol and subsequently to the necessary organelles (Portnoy et al., 2000). In contrast, Smf3p is present and stable at the vacuolar membrane independent of the metal availability in the surroundings (Portnoy et al., 2002; Portnoy et al., 2000). The three scenarios are summarized and represented in Figure 4.
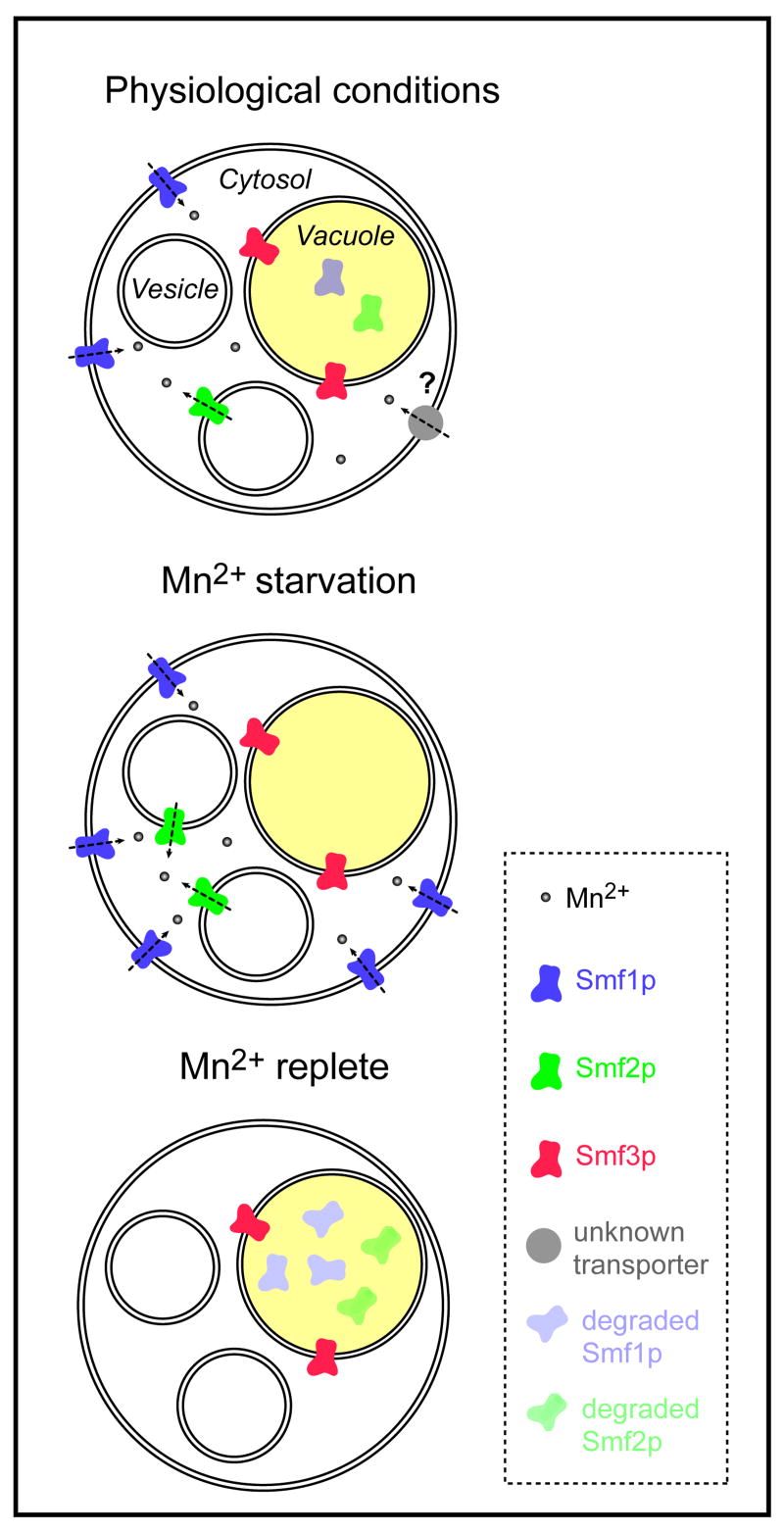
Figure 4. Localization of DMT1 isoforms in yeast upon environmental Mn concentration changes.
The yeast genome expresses three DMT1 isoforms: Smf1p, Smf2p and Smf3p. Smf1p is the only form localized at the plasma membrane and is probably responsible for most of the Mn uptake from the environment. Smf2p is localized in intracellular compartments but undetectable at the plasma membrane or at the vacuolar membrane. Smf3p is stably localized at the vacuole. Smf1p and Smf2p levels increase with Mn depletion, whereas in replete conditions, both Smf1p and Smf2p are targeted to the vacuole for degradation. In contrast, neither SMF3 expression levels nor Smf3p seem affected upon Mn concentration changes although they are sensitive to Fe levels. Knockout mutants of the three SMF genes are still able to take up Mn, suggesting the existence of an alternative transporter for Mn uptake from the environment. This figure is modified from Portnoy et al., 2000 and 2002.
DMT1 transport of Mn in the mammalian system
Although the use of S. cerevisiae provides a promising tool for the discovery of the role of DMT1 in the cellular uptake of Mn, it is believed that the exact mechanism for Mn regulation in the mammalian system involves a more extensive and sophisticated network. One of the earlier models that researchers used to study the metal delivery function of DMT1 in mammals was the b/b rat, which exhibits microcytic hypochromic anemia and systemic iron deficiency due to the disruption of DMT1. Hence, the functional consequences of this mutation in the b/b rat has been examined extensively in relation to Fe homeostasis. Since previous studies showed that both Fe and Mn bind to the TfR during intestinal absorption (Rossander-Hulten et al., 1991; Thomson et al., 1971), it was hypothesized that Mn and Fe may also compete for transport through DMT1. Hence, the b/b rat is considered currently the perfect mammalian model for studying Mn uptake.
Current research on the significance of DMT1 in Mn trafficking is both limited and controversial. Earlier studies have emphasized the possible function of DMT1 in Mn transport using b/b rats. In 1997, Chua and Morgan examined Mn metabolism as a function of uptake by reticulocytes and several organs, together with absorption from the duodenum and plasma clearance in control Wistar rats (+/+), heterozygous (+/b) rats and homozygous b/b rats. In general, Mn uptake by reticulocytes and in some organs, such as the kidney, brain and femurs, along with absorption from the duodenum, is significantly reduced in b/b rats as compared to +/b or +/+ rats. Furthermore, plasma clearance of Mn is much faster than that of Fe (Chua et al., 1997). These results suggest that b/b rats display impaired Mn metabolism due to a defect in Mn uptake and transport caused by a mutation in DMT1. Similar findings supported by Knopfel and Garrick present a direct link between DMT1 and a dysfunction in activity that accounts for the uptake and the transport of Mn ions into the small-intestinal membrane vesicle (Knopfel et al., 2005). The G185R mutation in b/b rats causes a complete disruption of DMT1 transport activity of Mn across the small-intestinal tissue, which was not observed in heterozygous +/b rats or Wistar rats. Both of these studies reveal the importance of functional DMT1, which is mandatory for the proper transport of Mn. Additionally, this theory was proven valid in olfactory Mn uptake. A study examining the contribution of olfactory DMT1 in Mn uptake showed that nasal absorption of Mn is significantly reduced in b/b rats and the protein levels of olfactory DMT1 were elevated in iron-deficient rats, suggesting the importance of DMT1 in olfactory Mn absorption that is dependent upon the iron status (Thompson et al., 2007).
However, the absolute requirement of DMT1 in Mn uptake has been contradicted by Crossgrove and Yokel in an in situ brain perfusion study which shows that, despite the hematological parameters and spleen/body weight variation among b/b, +/b, and +/+ rats, brain Mn uptake is not significantly different among these three rat strains. The authors suggest that Mn is distributed across the BBB into the brain by processes that are independent of DMT1 (Crossgrove et al., 2004). However, one has to take into account that there is a potential for high adaptation and compensation due to the DMT1 deficiency. To support this theory, further research is warranted.
Possible DMT1 transport of Mn in other systems
The use of molecular biology gave rise to the idea that other model organisms, such as Drosophila, honeybees and C. elegans may have some similar functional DMT1 homologues. The DMT1 orthologues of the fruit fly and nematode contain 12 TMD and share the same “consensus transport sequence” as the vertebrate DMT1, though the −IRE/+IRE system appears specific to vertebrates (Figure 2). In 1995, a screening for genes that influence responsiveness to stimuli discovered the Drosophila homologue of mammalian NRAMP, known as malvolio (mvl), and sequence homology indicated a 65% identity with human NRAMP (Rodrigues et al., 1995). Mutation in mvl showed reduced responsiveness to sucrose, which can be fully recovered by supplementation with Mn (Orgad et al., 1998) or expression of human NRAMP proteins (D'Souza et al., 1999). This suggests that the MVL protein is involved in taste perception in Drosophila and acts as a cation transporter similar to DMT1. Evidence also showed that this taste behavior holds true for honeybees, Apis mellifera. An increase in brain mvl expression leads to an elevation in Mn transport, sucrose responsiveness and, ultimately, division of labor within the colonies (Ben-Shahar et al., 2004).
In the nematode, C. elegans, there are three DMT1 isoforms: SMF-1, SMF-2 and SMF-3. Although none of the isoforms is fully characterized, expressions of C. elegans' smf-1 or smf-3 appear to rescue the hypersensitivity to EGTA in yeast smf-1/smf-2 mutants, implying that they both encode cation transporters with overlapping, if not identical, substrate specificity (Labrousse et al., 2000). Recently, a high-throughput in vivo analysis using green fluorescent protein (GFP) fusions generating spatial and temporal tissue expression profiles found that smf-3 is expressed in the intestine, suggesting that a likely route of Mn absorption in this species is via ingestion (Hunt-Newbury et al., 2007).
Conclusions
In summary, we have just begun to scratch the surface in examining how various types of living organisms effectively cope with metals that are both toxic and essential for growth. For each metal that enters the cell, a complex system of transport pathways is activated, and the exact machinery involved is determined by the organism's requirement in relation to its environment. The findings presented in this review provide some insight into the means that cells, yeast, rodents and possibly humans use to accumulate Mn via DMT1. The use of simple organisms such as Drosophila, honeybees and C.elegans that possess homologues of DMT1 has recently drawn increasing interest for discovering and defining the exact pathway involved in Mn uptake that may be common to human beings.
Future Perspectives
Due to some of the contradictions regarding the contribution of DMT1 in the trafficking of Mn, additional research is necessary to further investigate the precise route of Mn uptake and the defined mechanism through which DMT1 facilitates this process. Listed below are four main points that need to be addressed. First, due to the selective vulnerability of certain areas of the brain, it is imperative to explore the reason for the specific localization of DMT1 within the brain and the functional consequences for this specificity. Second, although it is logical to hypothesize that sites at which Mn is accumulated are areas of injury, accumulation does not necessarily confer toxicity. Therefore, further research is required to investigate the direct link of between Mn accumulation and its downstream target(s), as well as the associated clinical manifestations. Thirdly, one should also consider possible compensatory Mn export mechanisms during excessive accumulation that may alleviate tissue Mn burdens and Mn's toxic effect. These may include rescue pathways that are only specific to certain areas of the brain in order to overcome the negative effects of Mn overexposure. To date, there are no studies on mechanisms associated with the extrusion of Mn, and it remains unknown whether Mn shares common transporters with Fe in exiting cells. Lastly, it is essential to acknowledge the absence of research on the functional role of DMT1 in specific organs. Better understanding on the exact transport mechanism of DMT1 will be beneficial for studying the consequences of Mn toxicity. Although it is well established that DMT1 transports metals along with a proton, a transporter can function as a channel, a pump or a carrier. Thus, studying the 3D structure of DMT1 under various conditions (close and open states) using crystallography will undoubtedly facilitate resolutions to some of these issues.
Acknowledgments
This review was partially supported by grants from NIEHS 10563 and DoD W81XWH-05-1-0239 (MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addess KJ, Basilion JP, Klausner RD, Rouault TA, Pardi A. Structure and dynamics of the iron responsive element RNA: implications for binding of the RNA by iron regulatory binding proteins. J Mol Biol. 1997;274(1):72–83. doi: 10.1006/jmbi.1997.1377. [DOI] [PubMed] [Google Scholar]
- Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol. 1999;31(10):991–4. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108 3:429–32. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15(3):333–40. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33(3):345–9. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58(2):730–5. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Shanker G, Erikson K, Yang J, Mutkus LA. The uptake of manganese in brain endothelial cultures. Neurotoxicology. 2002;23(2):165–8. doi: 10.1016/s0161-813x(02)00056-6. [DOI] [PubMed] [Google Scholar]
- Beaumont C, Delaunay J, Hetet G, Grandchamp B, de Montalembert M, Tchernia G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood. 2006;107(10):4168–70. doi: 10.1182/blood-2005-10-4269. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y, Dudek NL, Robinson GE. Phenotypic deconstruction reveals involvement of manganese transporter malvolio in honey bee division of labor. J Exp Biol. 2004;207(Pt 19):3281–8. doi: 10.1242/jeb.01151. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66(6):1198–207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44(9):1583–6. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93(12):4406–17. [PubMed] [Google Scholar]
- Cellier M, Prive G, Belouchi A, Kwan T, Rodrigues V, Chia W, Gros P. Nramp defines a family of membrane proteins. Proc Natl Acad Sci U S A. 1995;92(22):10089–93. doi: 10.1073/pnas.92.22.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol [B] 1997;167(5):361–9. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- Cohen A, Nelson H, Nelson N. The family of SMF metal ion transporters in yeast cells. J Biol Chem. 2000;275(43):33388–94. doi: 10.1074/jbc.M004611200. [DOI] [PubMed] [Google Scholar]
- Courville P, Chaloupka R, Cellier MF. Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters. Biochem Cell Biol. 2006;84(6):960–78. doi: 10.1139/o06-193. [DOI] [PubMed] [Google Scholar]
- Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25(3):451–60. doi: 10.1016/j.neuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- D'Souza J, Cheah PY, Gros P, Chia W, Rodrigues V. Functional complementation of the malvolio mutation in the taste pathway of Drosophila melanogaster by the human natural resistance-associated macrophage protein 1 (Nramp-1) J Exp Biol. 1999;202(Pt 14):1909–15. doi: 10.1242/jeb.202.14.1909. [DOI] [PubMed] [Google Scholar]
- Davidsson L, Lonnerdal B, Sandstrom B, Kunz C, Keen CL. Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J Nutr. 1989;119(10):1461–4. doi: 10.1093/jn/119.10.1461. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–28. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dorman DC, McManus BE, Parkinson CU, Manuel CA, McElveen AM, Everitt JI. Nasal toxicity of manganese sulfate and manganese phosphate in young male rats following subchronic (13-week) inhalation exposure. Inhal Toxicol. 2004;16(67):481–8. doi: 10.1080/08958370490439687. [DOI] [PubMed] [Google Scholar]
- Eide DJ. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu Rev Nutr. 1998;18:441–69. doi: 10.1146/annurev.nutr.18.1.441. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Aschner M. The importance of glutamate, glycine, and gamma-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol. 2005;204(3):343–54. doi: 10.1016/j.taap.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95(3):1148–53. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102(5):1884–92. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. A Manganese-Enhanced Diet Alters Brain Metals and Transporters in the Developing Brain. Toxicol Sci. 2006;92(2):516–525. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16(1):41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J Exp Med. 1999;189(5):831–41. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25(2):514–25. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–8. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70(1):171–80. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- Huang E, Ong WY, Connor JR. Distribution of divalent metal transporter-1 in the monkey basal ganglia. Neuroscience. 2004;128(3):487–96. doi: 10.1016/j.neuroscience.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Hunt-Newbury R, Viveiros R, Johnsen R, Mah A, Anastas D, Fang L, Halfnight E, Lee D, Lin J, Lorch A, McKay S, Okada HM, Pan J, Schulz AK, Tu D, Wong K, Zhao Z, Alexeyenko A, Burglin T, Sonnhammer E, Schnabel R, Jones SJ, Marra MA, Baillie DL, Moerman DG. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 2007;5(9):e237. doi: 10.1371/journal.pbio.0050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolascon A, d'Apolito M, Servedio V, Cimmino F, Piga A, Camaschella C. Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2) Blood. 2006;107(1):349–54. doi: 10.1182/blood-2005-06-2477. [DOI] [PubMed] [Google Scholar]
- Jensen LT, Ajua-Alemanji M, Culotta VC. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem. 2003;278(43):42036–40. doi: 10.1074/jbc.M307413200. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Joshi PG, Joshi NB. Calcium sequestering ability of mitochondria modulates influx of calcium through glutamate receptor channel. Neurochem Res. 2000;25(12):1527–36. doi: 10.1023/a:1026602100160. [DOI] [PubMed] [Google Scholar]
- Knopfel M, Zhao L, Garrick MD. Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry. 2005;44(9):3454–65. doi: 10.1021/bi048768+. [DOI] [PubMed] [Google Scholar]
- Lam-Yuk-Tseung S, Camaschella C, Iolascon A, Gros P. A novel R416C mutation in human DMT1 (SLC11A2) displays pleiotropic effects on function and causes microcytic anemia and hepatic iron overload. Blood Cells Mol Dis. 2006;36(3):347–54. doi: 10.1016/j.bcmd.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Lam-Yuk-Tseung S, Mathieu M, Gros P. Functional characterization of the E399D DMT1/NRAMP2/SLC11A2 protein produced by an exon 12 mutation in a patient with microcytic anemia and iron overload. Blood Cells Mol Dis. 2005a;35(2):212–6. doi: 10.1016/j.bcmd.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lam-Yuk-Tseung S, Touret N, Grinstein S, Gros P. Carboxyl-terminus determinants of the iron transporter DMT1/SLC11A2 isoform II (-IRE/1B) mediate internalization from the plasma membrane into recycling endosomes. Biochemistry. 2005b;44(36):12149–59. doi: 10.1021/bi050911r. [DOI] [PubMed] [Google Scholar]
- Lee PL, Gelbart T, West C, Halloran C, Beutler E. The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol Dis. 1998;24(2):199–215. doi: 10.1006/bcmd.1998.0186. [DOI] [PubMed] [Google Scholar]
- Liao SL, Chen CJ. Manganese stimulates stellation of cultured rat cortical astrocytes. Neuroreport. 2001;12(18):3877–81. doi: 10.1097/00001756-200112210-00004. [DOI] [PubMed] [Google Scholar]
- Liu XF, Culotta VC. Mutational analysis of Saccharomyces cerevisiae Smf1p, a member of the Nramp family of metal transporters. J Mol Biol. 1999a;289(4):885–91. doi: 10.1006/jmbi.1999.2815. [DOI] [PubMed] [Google Scholar]
- Liu XF, Culotta VC. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J Biol Chem. 1999b;274(8):4863–8. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- Lucaciu CM, Dragu C, Copaescu L, Morariu VV. Manganese transport through human erythrocyte membranes. An EPR study. Biochim Biophys Acta. 1997;1328(2):90–8. doi: 10.1016/s0005-2736(97)00039-4. [DOI] [PubMed] [Google Scholar]
- Luk EE, Culotta VC. Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem. 2001;276(50):47556–62. doi: 10.1074/jbc.M108923200. [DOI] [PubMed] [Google Scholar]
- Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res. 1999;56(2):113–22. doi: 10.1002/(SICI)1097-4547(19990415)56:2<113::AID-JNR1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, Divoky V, Prchal JT. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood. 2005;105(3):1337–42. doi: 10.1182/blood-2004-07-2966. [DOI] [PubMed] [Google Scholar]
- Murphy VA, Wadhwani KC, Smith QR, Rapoport SI. Saturable transport of manganese(II) across the rat blood-brain barrier. J Neurochem. 1991;57(3):948–54. doi: 10.1111/j.1471-4159.1991.tb08242.x. [DOI] [PubMed] [Google Scholar]
- Nelson N. Metal ion transporters and homeostasis. Embo J. 1999;18(16):4361–71. doi: 10.1093/emboj/18.16.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Y. Site-directed mutagenesis investigation of coupling properties of metal ion transport by DCT1. Biochim Biophys Acta. 2007;1778(1):334–41. doi: 10.1016/j.bbamem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann N Y Acad Sci. 2004;1012:209–23. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Orgad S, Nelson H, Segal D, Nelson N. Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J Exp Biol. 1998;201(Pt 1):115–20. doi: 10.1242/jeb.201.1.115. [DOI] [PubMed] [Google Scholar]
- Pfeifer GD, Roper JM, Dorman D, Lynam DR. Health and environmental testing of manganese exhaust products from use of methylcyclopentadienyl manganese tricarbonyl in gasoline. Sci Total Environ. 2004;334-335:397–408. doi: 10.1016/j.scitotenv.2004.04.043. [DOI] [PubMed] [Google Scholar]
- Pinner E, Gruenheid S, Raymond M, Gros P. Functional complementation of the yeast divalent cation transporter family SMF by NRAMP2, a member of the mammalian natural resistance-associated macrophage protein family. J Biol Chem. 1997;272(46):28933–8. doi: 10.1074/jbc.272.46.28933. [DOI] [PubMed] [Google Scholar]
- Portnoy ME, Jensen LT, Culotta VC. The distinct methods by which manganese and iron regulate the Nramp transporters in yeast. Biochem J. 2002;362(Pt 1):119–24. doi: 10.1042/0264-6021:3620119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy ME, Liu XF, Culotta VC. Saccharomyces cerevisiae expresses three functionally distinct homologues of the nramp family of metal transporters. Mol Cell Biol. 2000;20(21):7893–902. doi: 10.1128/mcb.20.21.7893-7902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JE. Manganese oxide minerals: crystal structures and economic and environmental significance. Proc Natl Acad Sci U S A. 1999;96(7):3447–54. doi: 10.1073/pnas.96.7.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priwitzerova M, Nie G, Sheftel AD, Pospisilova D, Divoky V, Ponka P. Functional consequences of the human DMT1 (SLC11A2) mutation on protein expression and iron uptake. Blood. 2005;106(12):3985–7. doi: 10.1182/blood-2005-04-1550. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1987;67(3):858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61(2):509–17. doi: 10.1111/j.1471-4159.1993.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Ressler T, Wong J, Roos J. Manganese speciation in exhaust particulates of automobiles using MMT-containing gasoline. J Synchrotron Radiat. 1999;6(Pt 3):656–8. doi: 10.1107/S0909049598015623. [DOI] [PubMed] [Google Scholar]
- Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem. 2002;277(14):12302–9. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- Rodrigues V, Cheah PY, Ray K, Chia W. malvolio, the Drosophila homologue of mouse NRAMP-1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behaviour. Embo J. 1995;14(13):3007–20. doi: 10.1002/j.1460-2075.1995.tb07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin H, Mathee A, Levin J, Theodorou P, Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ Res. 2005;97(1):93–9. doi: 10.1016/j.envres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rossander-Hulten L, Brune M, Sandstrom B, Lonnerdal B, Hallberg L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991;54(1):152–6. doi: 10.1093/ajcn/54.1.152. [DOI] [PubMed] [Google Scholar]
- Sacher A, Cohen A, Nelson N. Properties of the mammalian and yeast metal-ion transporters DCT1 and Smf1p expressed in Xenopus laevis oocytes. J Exp Biol. 2001;204(Pt 6):1053–61. doi: 10.1242/jeb.204.6.1053. [DOI] [PubMed] [Google Scholar]
- Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92(6):2157–63. [PubMed] [Google Scholar]
- Supek F, Supekova L, Nelson H, Nelson N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci U S A. 1996;93(10):5105–10. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi M, Yoshimori T, Yamaguchi K, Yoshida T, Kishi F. Human NRAMP2/DMT1, which mediates iron transport across endosomal membranes, is localized to late endosomes and lysosomes in HEp-2 cells. J Biol Chem. 2000;275(29):22220–8. doi: 10.1074/jbc.M001478200. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41(1):79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem. 2000;275(2):1023–9. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. Faseb J. 2007;21(1):223–30. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AB, Olatunbosun D, Valverg LS. Interrelation of intestinal transport system for manganese and iron. J Lab Clin Med. 1971;78(4):642–55. [PubMed] [Google Scholar]
- Touret N, Furuya W, Forbes J, Gros P, Grinstein S. Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrin receptor. J Biol Chem. 2003;278(28):25548–57. doi: 10.1074/jbc.M212374200. [DOI] [PubMed] [Google Scholar]
- Vidal S, Belouchi AM, Cellier M, Beatty B, Gros P. Cloning and characterization of a second human NRAMP gene on chromosome 12q13. Mamm Genome. 1995;6(4):224–30. doi: 10.1007/BF00352405. [DOI] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res. 2006;1097(1):1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Ong WY, Connor JR. A light and electron microscopic study of the iron transporter protein DMT-1 in the monkey cerebral neocortex and hippocampus. J Neurocytol. 2001;30(4):353–60. doi: 10.1023/a:1014464514793. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul. 1984;24:153–69. doi: 10.1016/b978-0-12-152824-9.50021-6. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Denman RB, Roby WG. Glutamine synthetase from ovine brain is a manganese(II) enzyme. Biochemistry. 1982;21(25):6389–96. doi: 10.1021/bi00268a011. [DOI] [PubMed] [Google Scholar]
- West AH, Clark DJ, Martin J, Neupert W, Hartl FU, Horwich AL. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992;267(34):24625–33. [PubMed] [Google Scholar]