Abstract
Non-sexual social bonding between adult mammals remains poorly understood, despite its importance in many species. Female meadow voles are territorial and nest alone in long summer day lengths when circulating estradiol concentrations are high, but cohabit in groups in short winter photoperiods when estradiol secretion is low. The influence of day length and estradiol on same-sex huddling behavior was assessed in adult female pairs housed together in long day lengths (LDs) or short day lengths (SDs) from weaning. The behavior of intact, ovariectomized, and estradiol-treated ovariectomized females from each photoperiod was assessed during 3 hour partner preference tests. Intact SD voles, unlike intact LD voles, spent the majority of the test in proximity to their cage mates. Estradiol treatment of SD voles significantly reduced time spent huddling with the partner. Neither ovariectomy nor estradiol treatment significantly affected the amount of time LD females spent in contact with their partners. Low estradiol availability is therefore a necessary but not sufficient condition for maintenance of high levels of huddling. These results establish that ovarian hormones interact with photoperiod to affect same-sex social behavior.
Keywords: social behavior, partner preference, prosocial behavior, day length, estradiol, body temperature, rodent, vole
Introduction
Across the animal kingdom, affiliative social relationships exist between individuals and their parents, offspring, mates, and non-related conspecifics. In the last two decades, neurobiological investigations of parental behavior (Numan and Insel, 2003) and partner preference formation between mates (Young and Wang, 2004; Lim and Young, 2006) have yielded important insights into these behaviors. Little is known, however, about the factors involved in non-sexual prosocial behavior (Tang-Martinez, 2003; Goodson et al., 2006). Same-sex social relationships are a common feature of social species, and in many cases form the basis for group living. In this study we focused on variation in same-sex social behavior of female meadow voles (Microtus pennsylvanicus).
Meadow voles, unlike prairie voles, do not form socially monogamous partnerships (Getz, 1972, Boonstra et al., 1993). In common with many rodents from temperate latitudes, meadow voles are territorial and inhabit separate burrows throughout the summer when they are reproductively active. During fall and winter months, however, they share nests (McShea and Madison, 1984). Groups typically begin with a female and her offspring, who are then joined by immigrant males (Madison et al., 1984). By late December to early January, migration subsequent to predation leads to social constellations that no longer represent family lineages. These mixed-sex groups consist of 3–10 voles that sleep in clusters of 2–3 (Madison and McShea, 1987). Tests of dyadic interactions between field caught voles indicate that males and females are tolerant of both nestmates and strangers during winter months when the gonads are regressed or not yet developed. By spring, aggression towards strangers increases in both sexes, concurrent with gonadal development (McShea, 1990). Spring social groups often cohere through the first reproductive period, but they are closed to the immigration of new members (McShea and Madison, 1984; McShea, 1990).
Variations in day length in laboratory studies trigger at least some of the changes in behavior observed seasonally in the field (Ferkin and Seamon, 1987). In long day lengths (LDs), female meadow voles prefer the odors of males over those of females, consistent with summer breeding. In short day lengths (SDs), however, females prefer the odors of other females over their own odor or those of males (Ferkin and Zucker, 1991). The LD female’s preference for male odors is estradiol-dependent and eliminated by ovariectomy, whereas the SD female’s preference for female odors is affected neither by ovariectomy nor estradiol (E) (Ferkin and Zucker, 1991).
Same-sex female prosocial behavior in SDs extends to social interactions as well as olfactory preference. Female meadow voles housed in SDs form selective partner preferences for cohabitating females (Parker and Lee, 2003).
A variety of potential mechanisms may contribute to the differences in behavior between winter and summer phenotypes in the field. Day length, transduced by the hormone melatonin, leads to seasonal changes in physiology. Gonadal steroid secretion is markedly reduced in SD, and differences in brain receptor systems might alter responsiveness to estradiol and other hormones. In female prairie voles, oxytocin, interacting with dopamine in the nucleus accumbens, is critical for opposite sex social bonding (Liu and Wang, 2003). While meadow voles have a low density of oxytocin receptors in the nucleus accumbens (Insel and Shapiro, 1992), OT may also influence social behavior by acting in other brain regions such as the amygdala, lateral septum, or bed nucleus of the stria terminalis (discussed in Beery et al. 2008). Estradiol regulates oxytocin production in select brain areas (Breton and Zingg, 1997; Young et al, 1997; Patisaul et al. 2003), and oxytocin receptor distribution varies between sexually inexperienced meadow voles housed in LD and SD (Parker et al. 2001).
Alternatively, differences in social behavior could be independent of social motivation if temperature can fully predict huddling. In the field, the degree of social nesting in meadow voles increases with decreased ground-level temperature (Madison et al., 1984). Social tolerance in winter probably evolved at least in part for its thermoregulatory benefits, which have been demonstrated in voles and other rodents (for example Andrews et al. 1987, Andrews and Belknap, 1993, Kauffman et al., 2003). Some rodent species adjust their basal body temperature (Tb) in response to day length, independent of external temperature. For example, mean Tb of Siberian hamsters maintained at 23°C in SD is 0.7°C lower than mean Tb of LD hamsters housed at the same temperature (Heldmaier et al., 1989). Thus, even at uniform external temperature, Tb could potentially govern day length dependent differences in huddling.
The present study assessed whether seasonal differences in huddling behavior observed in the field translate to measurable differences in partner preference test behavior of LD- and SD-housed female voles. We evaluated whether the seasonal decrease in ovarian hormone secretion associated with the transition from LDs to SDs promotes increased same-sex social contact; specifically, we assessed whether the decline in estradiol secretion consequent to ovariectomy is permissive of same-sex partner preference formation in LD female voles. The effect of estradiol supplementation on same-sex partner preferences of SD females was also determined. Finally, we monitored body temperature in a separate group of voles to determine whether or not seasonal variation in Tb between voles housed at a uniform temperature in SD and LD might account for differences in huddling behavior.
Materials and Methods
Animals
Meadow voles that formed the breeding stock of our colony were supplied by Michael Ferkin of the University of Memphis and paired in LDs (14:10 light:dark cycle). Female offspring were weaned at 19–20 days of age as same-sex pairs and transferred to SDs (10 h light/day) or maintained in LDs. Dark onset was 17:00 PDT in both photoperiods. Voles were housed in clear plastic cages (48 × 25 × 15 cm) furnished with pine bedding, cotton nesting material (Nestlets), and opaque plastic refuge tubes large enough to accommodate two voles. Food (mouse chow no. 5015, Purina Mills, St. Louis MO) and tap water were available ad libitum. Ambient temperature was 21 ± 1°C. Animal care and all experimental procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley.
Experimental Design and Timeline
60 pairs of females were assigned to six treatment groups (n=10 pairs/group). In each day length, voles were left intact (LDint and SDint), ovariectomized (LDovx and SDovx), or ovariectomized and treated with an estradiol-filled capsule (LDovxE and SDovxE). Voles that were not ovariectomized underwent sham ovariectomies; voles that did not receive an estradiol-filled capsule were treated with a blank capsule.
Ovariectomies and sham ovariectomies were performed on mature young females at 40 ± 5 days of age (as in Ferkin and Zucker, 1991) as described below. Twelve days later, voles were treated with a capsule containing crystalline estradiol or a blank control capsule. Capsules were constructed of Silastic tubing (ID 1.47 mm, OD 1.96 mm, Dow Corning, Midland, MI) plugged with Silicone and packed for a length of 5mm with estradiol (Sigma, St. Louis) or left empty (same treatment as in Ferkin and Zucker, 1991). Three weeks later at 70–80 days of age, voles underwent behavioral testing in a partner preference apparatus.
1–2 weeks after behavioral testing, animals were sacrificed and presence of the capsule was confirmed. In subsets of voles (2–3 per group), uteri were removed, trimmed just below the ovaries or point of ovariectomy, defatted with forceps, and weighed (± 0.1 mg).
Surgical Procedures
Ovariectomies and sham ovariectomies were performed under isoflurane anesthesia. A 0.1cc subcutaneous injection of Buprenorphine (.015mg/ml) was administered 5–10 min before surgery. The ovaries were accessed via two lateral incisions; non-dissolving suture was used to tie off the uterine horns just below the ovaries and the ovaries were removed. In sham procedures the ovaries were externalized and replaced in the body cavity. The muscle wall was closed with dissolvable sutures, and the skin was closed with non-dissolving sutures and wound clips.
Capsules were soaked in saline for 24 h prior to insertion, and implanted subcutaneously between the scapulae of isoflurane-anesthetized voles. The incision was closed with a wound clip and animals were ear-tagged for identification. All wound clips were removed within 2 weeks of placement.
Behavioral Testing
Behavioral tests were modeled on the partner-preference test developed in the laboratory of C. S. Carter (Carter et al., 1995), and were conducted in an apparatus consisting of three equal plastic cages (17cm × 28cm × 12.5cm): one neutral rear chamber connected by tubes (5cm diameter, 5cm length) to two front chambers. Before each test, one member of the test-pair was designated the focal (untethered) vole. The day before behavioral testing the focal vole was placed in the apparatus for 10 min, or until she explored each chamber at least once.
On the day of the behavioral test, the other member of the co-housed test-pair (the partner) and an unfamiliar vole from the same treatment condition (the stranger) were anesthetized briefly with isoflurane vapor and tethered in separate front chambers. Tethers consisted of a 10 cm nylon tie (Radio Shack) attached by a swivel to flexible braided steel fishing leader (South Bend Sporting Goods, Northfield IL). Tethers were affixed to the chamber lids and permitted movement of the tethered vole throughout half the chamber. The positions of the partner and stranger (left versus right chamber) were alternated between tests of each type. Tethered voles were acclimated to the chamber for 5 min before the focal animal was placed in the neutral (rear) chamber and allowed to move freely for the duration of the 3 h test. Apparatuses were washed thoroughly with soap and water after each acclimation or behavioral test.
Time-lapse footage of behavioral tests was recorded at 1 frame/sec using a video camera (Sony DCR-TRV900) attached to a Macintosh laptop running Gawker version 0.8 (Phil Piwonka). Continuous video was recorded simultaneously on mini-DV tapes (Sony DVC-60), and archived for reference. Experimenters were absent from the test room during recording of social behavior.
Time-lapse video files were scored by an experimenter using the program Intervole Timer (Annaliese Beery) to record counts and durations of presence in each chamber and of huddling. Huddling was defined as side-by-side contact of the focal and tethered voles. Descriptions of interactions were recorded as annotations at the end of each file. Scoring was conducted without any knowledge of treatment groups.
Data from four pairs were removed from consideration either because one of the tethered voles became free of its restraint during behavioral testing or because upon autopsy the estradiol capsule was determined to have been extruded. Additional pairs were added to the study to achieve final sample sizes of 10/group.
Temperature monitoring
Body temperature was monitored in 6 LD and 6 SD voles at ~3 months of age. Voles were singly housed during the period of Tb monitoring. iButton temperature sensors (model DS1921H-F5#, Maxim Integrated Products, Sunnyvale, CA) were soaked in alcohol prior to implantation in the abdomen. Anesthesia and wound closure were as described for the ovariectomy procedure above. Tb readings were recorded at 15 min intervals for 2 weeks in each vole. Two iButton sensors measured room temperature in the LD and SD rooms.
Data analysis
Analysis within treatment groups: partner preference
Total time spent huddling with the partner versus stranger was compared within each treatment group using t-tests assuming unequal variances. Partner preference was inferred when the focal vole spent at least twice as much time in side-by-side contact with the familiar versus the unfamiliar vole (as in Insel et al., 1995, Parker et al., 2003).
Analysis between groups: treatment effects
Effects of day length (LD or SD) and surgical treatment (intact, OVX, or OVX+E) were analyzed by 2-way ANOVA. Because of a significant interaction between day length and surgical treatment, each day length and surgical combination was analyzed by ANOVA followed by Dunnett’s test of difference from the control group (LD intact females). Analyses were performed on the total time spent in the chamber with another vole and on total time huddling with another vole.
Statistical analyses were performed using JMP 5.1 (SAS Institute Inc., Cary, NC). Means ± SEM are reported throughout.
Results
Day length and social behavior
There was a significant main effect of day length on social behavior (F5,54=10.30, P < 0.005); LD voles generally spent less time huddling or in another vole’s chamber than did SD voles. LDint and SDint females spent an average of 5% versus 41%, respectively, of each 3 h test huddling with either tethered vole, and 14% versus 76%, respectively, in one of the chambers containing a vole (table 1). These group differences were both significant (P < 0.005, Dunnet’s test). Investigatory activity, measured by the number of entries into an adjacent chamber, was greater in SD than LD groups during the 3h test (134 ± 17 and 82 ± 16, respectively, P < 0.05).
Table 1.
Total chamber and huddling time by treatment group
| Group abbrev | % of test spent in occupied chambers | % of test spent huddling |
|---|---|---|
| LDint | 14±4 | 5±3 |
| LDovx | 29±10 | 16±7 |
| LDovxE | 26±10 | 14±6 |
| SDint | 76±5 | 41±6 |
| SDovx | 59±11 | 41±11 |
| SDovxE | 25±9 | 10±6 |
Surgical and hormonal effects
The interaction between day length and surgery was significant (P < 0.05); consequently, all combinations of day length and surgical/hormonal treatments were analyzed by one-way ANOVA.
In LDs, there was no effect of surgical or hormonal treatment on behavior in the partner preference test. The LDovx and LDovxE groups were indistinguishable from intact LD voles in total huddling time and total time spent in another vole’s chamber (Fig. 1A,B).
Fig 1.
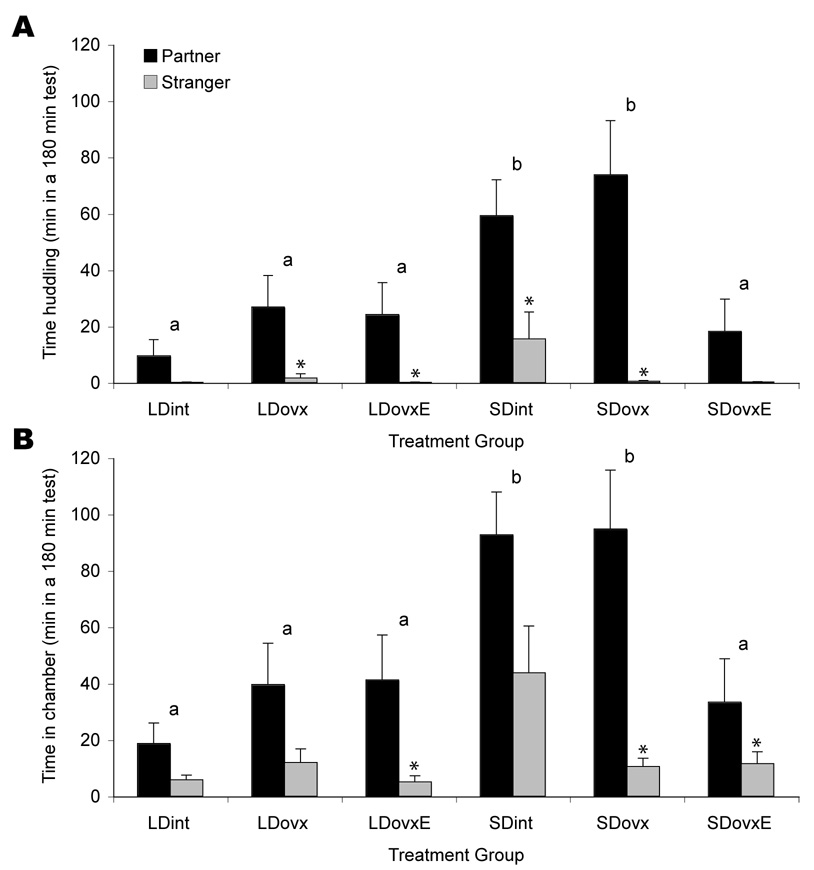
(A) Mean (± SEM) time focal females spent huddling with the partner (■) or the stranger ( ) during a 3 h test. (B) Mean (± SEM) time focal females spent in the same chamber as the partner or the stranger during a 3 h test. Asterisks denote significant differences within groups (between partners and strangers). Letters denote differences between treatment groups — groups labeled “a” are not different from LD intact voles, whereas groups labeled “b” are significantly different from LD intact animals (P <0.005, Dunnet’s test).
) during a 3 h test. (B) Mean (± SEM) time focal females spent in the same chamber as the partner or the stranger during a 3 h test. Asterisks denote significant differences within groups (between partners and strangers). Letters denote differences between treatment groups — groups labeled “a” are not different from LD intact voles, whereas groups labeled “b” are significantly different from LD intact animals (P <0.005, Dunnet’s test).
In SDs, both intact and ovariectomized voles spent significantly more time huddling as well as more time in vole-occupied chambers than did LD intact animals (P < 0.005 in each case, Dunnet’s test, Fig. 1A,B). SD voles treated with estradiol (SDovxE) did not differ from LD intact animals on either measure (P = 0.98 and 0.83, Dunnet’s test, Fig. 1A,B).
Uterine mass is a proxy for exposure to estradiol (Lundeen et al. 1997). Uterine mass varied with day length and surgical treatment (F5,7=39.83, P < 0.001). LDovx, SDovx, and SDint voles had lighter uteri than LDint, LDovxE and SDovxE voles (Tukey-Kramer test, Fig. 2A).
Fig 2.
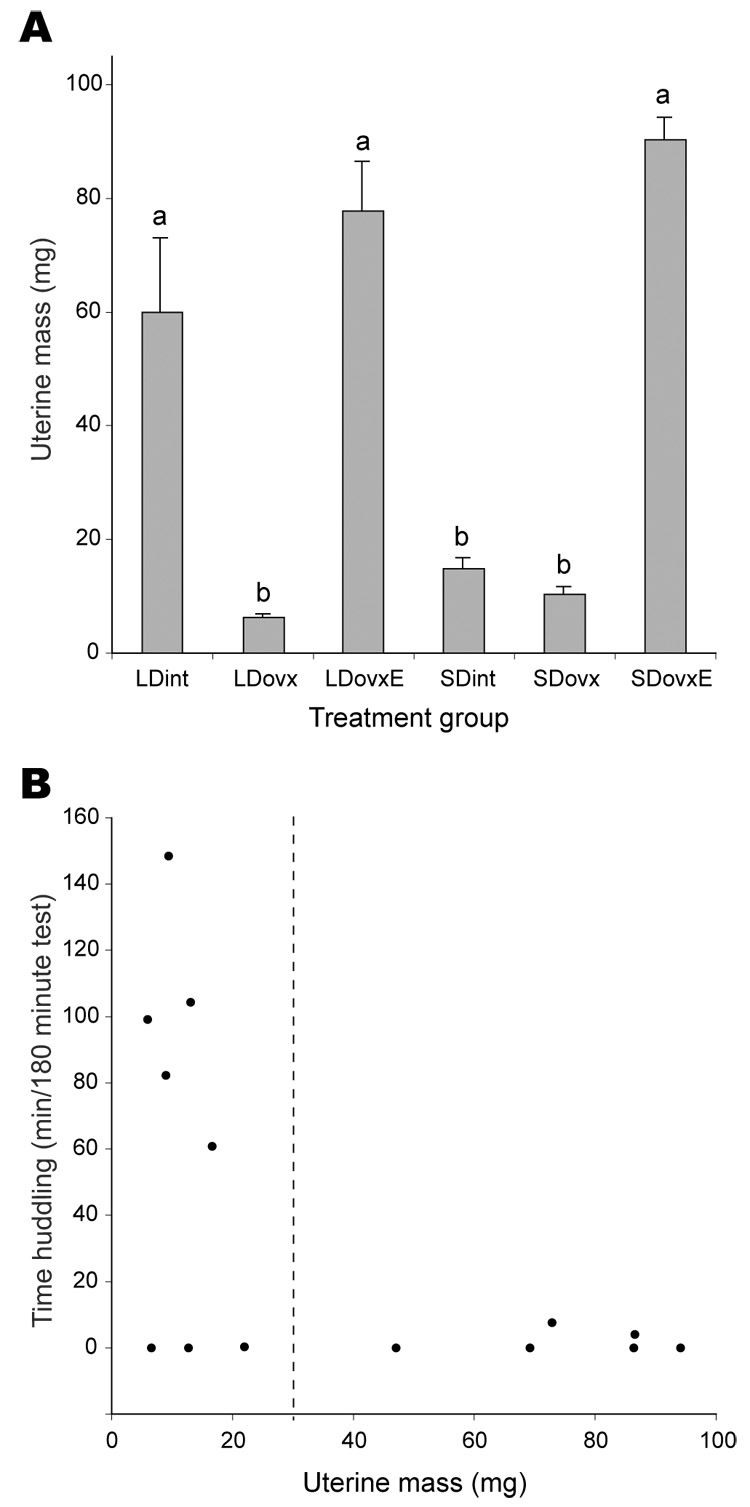
(A) Mean uterine mass (± SEM) for a subset of focal females from each treatment group (uterine mass is a proxy for, and is correlated with exposure to circulating estradiol). Groups labeled “a” are significantly different from those labeled “b” (Tukey-Kramer test). (B) Bivariate analysis of total huddling time by uterine mass. At low uterine masses (< 30 mg) social behavior was varied, whereas voles with high uterine masses exhibited little or no huddling behavior.
A plot of uterine mass against total huddling time across all treatment groups (Fig. 2B) indicates that no vole with uterine mass > 20 mg spent substantial time huddling. Low uterine mass was associated with a range of huddling durations (0 to 150 min) and did not predict huddling behavior, whereas high uterine mass was invariably associated with low huddling durations.
Partner preference
Voles in all treatment groups exhibited a partner preference, defined as at least twice as much time in contact with the partner as with the stranger. In all but the LDint group, the durations of chamber time or huddling time with the partner were significantly different from the respective durations with the stranger (Fig. 1 A,B). LD intact females also tended to huddle more with their partner than the stranger, but this effect was not significant (P = 0.13).
Although they spent more time with partners than with strangers, SD intact animals spent substantially more time in stranger compartments than did LD intact animals (P < 0.001). SD intact voles also spent more time huddling with a stranger than did LD intact voles (P < 0.01).
Partner placement in the right versus the left side of the apparatus did not affect time spent huddling with the partner (P = 0.74) or time spent in the partner’s chamber (P = 0.50).
Temperature rhythms
LD and SD voles manifested daily Tb fluctuations of approximately 1°C (Fig. 3). Despite Tb differences between LD and SD groups at some times of day, there was no significant difference between mean LD and SD Tb (P = 0.62), nor did these groups’ Tb differ during the 3 h social testing window (P = 0.82). Although the phasing of Tb rhythms was different in SDs than LDs when plotted in terms of clock time (Fig. 3A), re-plotting in terms of hours since light onset (03:00 in LD and 07:00 in SD) reveals substantially more similar Tb profiles during the common lights-on period (Fig. 3B). Both LD and SD voles exhibited striking ultradian variations in body temperature with periods of ~3h (cf Zubidat et al., 2007). At 80 days of age, no difference in body mass was detected between LD and SD housed female voles (P = 0.37).
Fig 3.
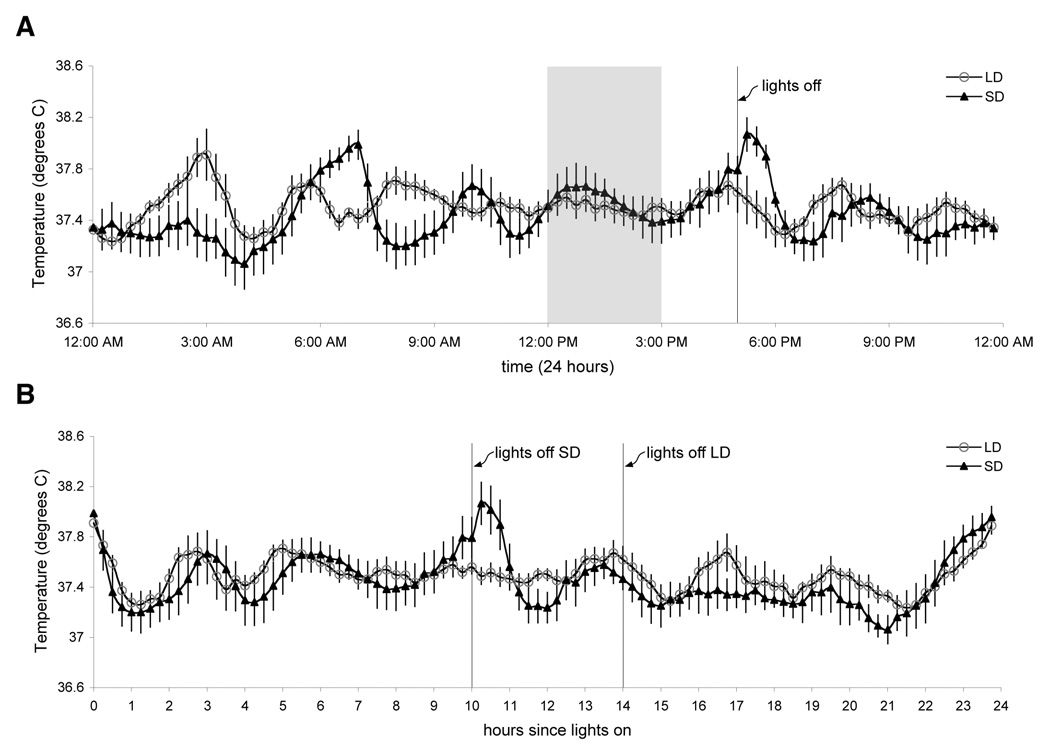
Rhythms in body temperature of LD and SD voles (n=6 females/group; mean ± SEM). (A) Body temperature as a function of clock time. The shaded area represents the time of social testing; groups did not differ in Tb during this interval. (B) Body temperature as a function of hours since light onset. In this view Tb rhythms in LD and SD are strikingly similar, with an ultradian period of ~3 hrs.
Discussion
The present study provides the first definitive evidence that day length affects same-sex huddling behavior of female meadow voles. Greater huddling behavior in intact SD than intact LD female voles was predicted on the basis of seasonal differences in territoriality and nest occupancy in field studies (McShea and Madison, 1984, Madison and McShea, 1987). We now show that this difference translates to differences in laboratory measures of same-sex social behavior.
Female voles in all groups, irrespective of day length or hormonal treatment, exhibited a partner preference for their cage-mates, while differing greatly in the total amount of time spent in contact with other animals during the preference test. This suggests that the experimental treatments caused differences in the levels of social motivation without altering the target of social behavior. This contrasts to results obtained in opposite-sex prairie voles, where social behavior in LD is always high, but changes from non-specific to specific affiliation with increased housing time or sexual activity (Williams et al., 1992).
Circulating estradiol had prominent effects on social behavior; groups with relatively high estradiol exposure, whether endogenous or exogenous in origin, uniformly spent little time huddling with same-sex conspecifics. Subcutaneous implantation of estradiol capsules does not alter the olfactory preferences of SD female meadow voles (Ferkin and Zucker, 1991), whereas similar treatments in the present study profoundly diminished SD huddling behavior to levels that did not differ from those of LD females. Seasonal effects of estradiol therefore appear to be trait-specific. Our tests were restricted to same-sex female voles, and therefore leave open the question of how ovarian secretions would affect opposite-sex huddling behavior.
When circulating estradiol was low, day length dictated social behavior, irrespective of estradiol concentrations; i.e., huddling did not increase substantially in ovariectomized LD voles and remained high in SD ovariectomized females. Differences in current exposure to ovarian hormone secretions are thus insufficient to account for all of the behavioral differences between LD and SD voles.
It is probable that the influence of day length on same-sex affiliative behavior is transduced by pineal melatonin secretion. Ferkin and Kile (1996) previously concluded that photoperiod-controlled differences in melatonin secretion mediate seasonal differences in meadow vole olfactory preferences. Photoperiod may affect female behavior by altering estradiol secretion, and/or by triggering seasonal changes in central nervous system physiology. Brain distribution of estrogen receptors (ER) and oxytocin receptors (OTR) may be particularly important for differences in social behaviors. ER-α density appears to be associated with social organization in male rodents (Cushing and Wynne-Edwards, 2006), and ER expression is associated with photoperiod dependent changes in aggressive behavior in Peromyscus (Trainor et al. 2007). In meadow voles, OTR binding is higher in the lateral septum, central amygdala, and lateral amygdala of naïve SD females than LD females (Parker et al., 2001). We are currently investigating the influence of day length and estradiol on OTR and ER distributions in female meadow voles.
Differences in SD and LD huddling behavior may be mediated by differences in social motivation, but they might also be proximately affected by differences in body temperature. Huddling behavior is responsive to variations in ambient temperature (Batchelder et al., 1983; Kauffman et al., 2003) and also affects Tb and metabolic rate (Andrews et al., 1987). SD-housed meadow voles eventually weigh less and consume less energy than LD voles (Dark and Zucker, 1985). Despite housing in constant ambient temperature, we considered that SD voles might respond to day length by lowering Tb, as is the case for Siberian hamsters (Heldmaier et al., 1989), thus triggering greater huddling for thermogenic purposes. This was not the case in the present study. Neither mean 24-h Tb, nor Tb during the 3 h interval during which behavioral tests were conducted, differed significantly between LD- and SD-voles tested at ~90 days of age. Body mass also did not differ at this age. Whereas energy savings from huddling may have shaped the evolution of increased huddling behavior in SDs, differences in Tb cannot account for the photoperiod-dependent differences in huddling behavior found in this study.
Huddling behavior of SD housed females in the laboratory may reflect increased prosocial motivation in winter-like photoperiods. Studies of arvicoline rodents have provided important insights into sociality, particularly in relation to mating systems (Curtis et al., 2007). Non-sexual social behavior is a common facet of social relationships in several taxa; additional studies of meadow voles may shed light on the means by which non-sexual relationships are promoted and maintained in mammals.
Acknowledgments
We are grateful to Michael Ferkin and to Zuoxin Wang for providing the meadow voles used to develop our colony, to David Routman for laboratory assistance and Chris Tuthill for laboratory management. Erica Maulhardt and Mae Barfeei assisted in the care and maintenance of breeding voles, and Joe Driscoll with behavioral scoring. This research was supported by NIH grant MH-61171 and a NDSEG fellowship to A. Beery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews RV, Phillips D, Makihara D. Metabolic and thermoregulatory consequences of social behaviors between Microtus townsendii. Comp Biochem Physiol A. 1987;87:345–348. doi: 10.1016/0300-9629(87)90133-2. [DOI] [PubMed] [Google Scholar]
- Andrews RV, Belknap RW. Season affects tolerance of cohabitation by deer mice. Physiol Behav. 1993;53:617–620. doi: 10.1016/0031-9384(93)90163-a. [DOI] [PubMed] [Google Scholar]
- Batchelder P, Kinney RO, Demlow L, Lynch CB. Effects of temperature and social interactions on huddling behavior in Mus musculus. Physiol Behav. 1983;31:97–102. doi: 10.1016/0031-9384(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Xia X, Pavone L. Mating system of the meadow vole, Microtus pennsylvanicus. Behav Ecol. 1993;4:83–89. [Google Scholar]
- Breton C, Zingg HH. Expression and region-specific regulation of the oxytocin receptor gene in rat brain. Endocrinology. 1997;138:1857–1862. doi: 10.1210/endo.138.5.5127. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang X. Neural regulation of social behavior in rodents. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological & evolutionary perspective. Chicago: University of Chicago Press; 2007. pp. 185–194. [Google Scholar]
- Cushing BS, Wynne-Edwards KE. Estrogen receptor-alpha distribution in male rodents is associated with social organization. J Comp Neurol. 2006;494:595–605. doi: 10.1002/cne.20826. [DOI] [PubMed] [Google Scholar]
- Dark J, Zucker I. Seasonal cycles in energy balance: regulation by light. Ann N Y Acad Sci. 1985;453:170–181. doi: 10.1111/j.1749-6632.1985.tb11809.x. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Kile JR. Melatonin treatment affects the attractiveness of the anogenital area scent in meadow voles (Microtus pennsylvanicus) Horm Behav. 1996;30:227–235. doi: 10.1006/hbeh.1996.0027. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Seamon O. Odor preference and social behavior in meadow voles, Microtus pennsylvanicus: seasonal differences. Can J Zool. 1987;65:2931–2937. [Google Scholar]
- Ferkin MH, Zucker I. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J Reprod Fertil. 1991;92:433–441. doi: 10.1530/jrf.0.0920433. [DOI] [PubMed] [Google Scholar]
- Getz LL. Social structure and aggressive behavior in a population of Microtus pennsylvanicus. J Mammal. 1972;53:310–317. [Google Scholar]
- Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmaier G, Steinlechner S, Ruf T, Wiesinger H, Klingenspor M. Photoperiod and thermoregulation in vertebrates: body temperature rhythms and thermogenic acclimation. J Biol Rhythms. 1989;4:251–265. [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Paul MJ, Butler MP, Zucker I. Huddling, locomotor, and nest-building behaviors of furred and furless Siberian hamsters. Physiol Behav. 2003;79:247–256. doi: 10.1016/s0031-9384(03)00115-x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lundeen SG, Carver JM, McKean ML, Winneker RC. Characterization of the ovariectomized rat model for the evaluation of estrogen effects on plasma cholesterol levels. Endocrinology. 1997;138:1552–1558. doi: 10.1210/endo.138.4.5083. [DOI] [PubMed] [Google Scholar]
- Madison DM, McShea W. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. Amer Zool. 1987;27:899–908. [Google Scholar]
- McShea WJ. Social tolerance and proximate mechanisms of dispersal among winter groups of meadow voles, Microtus pennsylvanicus. Anim Behav. 1990;39:346–351. [Google Scholar]
- Madison DM, Fitzgerald RW, McShea WJ. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav Ecol Sociobiol. 1984;15:9–17. [Google Scholar]
- McShea WJ, Madison DM. Communal nesting by reproductively active females in a spring population of Microtus pennsylvanicus. Can J Zool. 1984;62:344–346. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Parker KJ, Lee TM. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J Comp Psychol. 2003;117:283–289. doi: 10.1037/0735-7036.117.3.283. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Kinney LF, Lee TM. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115:1349–1356. [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- Tang-Martinez Z. Emerging themes and future challenges: Forgotten rodents, neglected questions. J Mammal. 2003;84:1212–1227. [Google Scholar]
- Trainor BC, Rowland MR, Nelson RJ. Photoperiod affects estrogen receptor alpha, estrogen receptor beta and aggressive behavior. Eur J Neurosci. 2007;26:207–218. doi: 10.1111/j.1460-9568.2007.05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol. 1997;9:859–865. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zubidat AE, Ben-Shlomo R, Haim A. Thermoregulatory and endocrine responses to light pulses in short-day acclimated social voles (Microtus socialis) Chronobiol Int. 2007;24:269–288. doi: 10.1080/07420520701284675. [DOI] [PubMed] [Google Scholar]


