Abstract
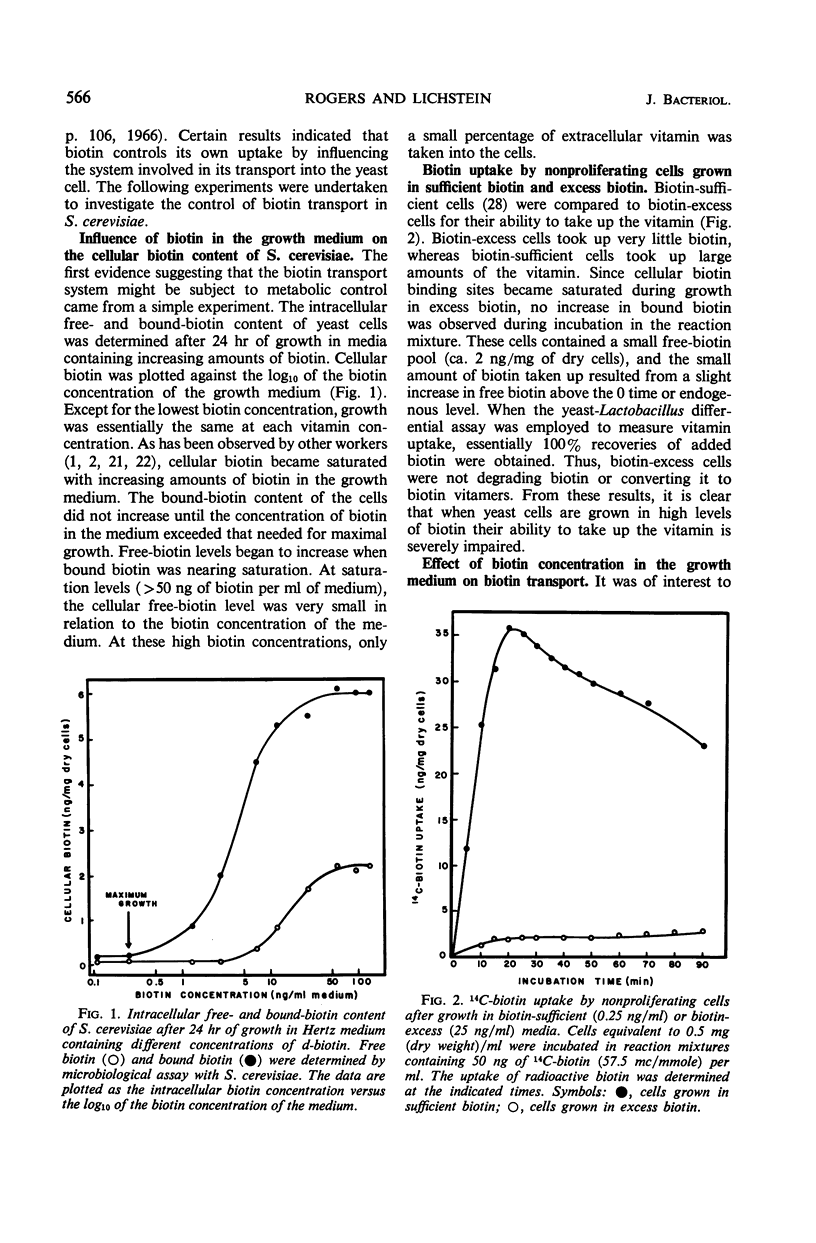
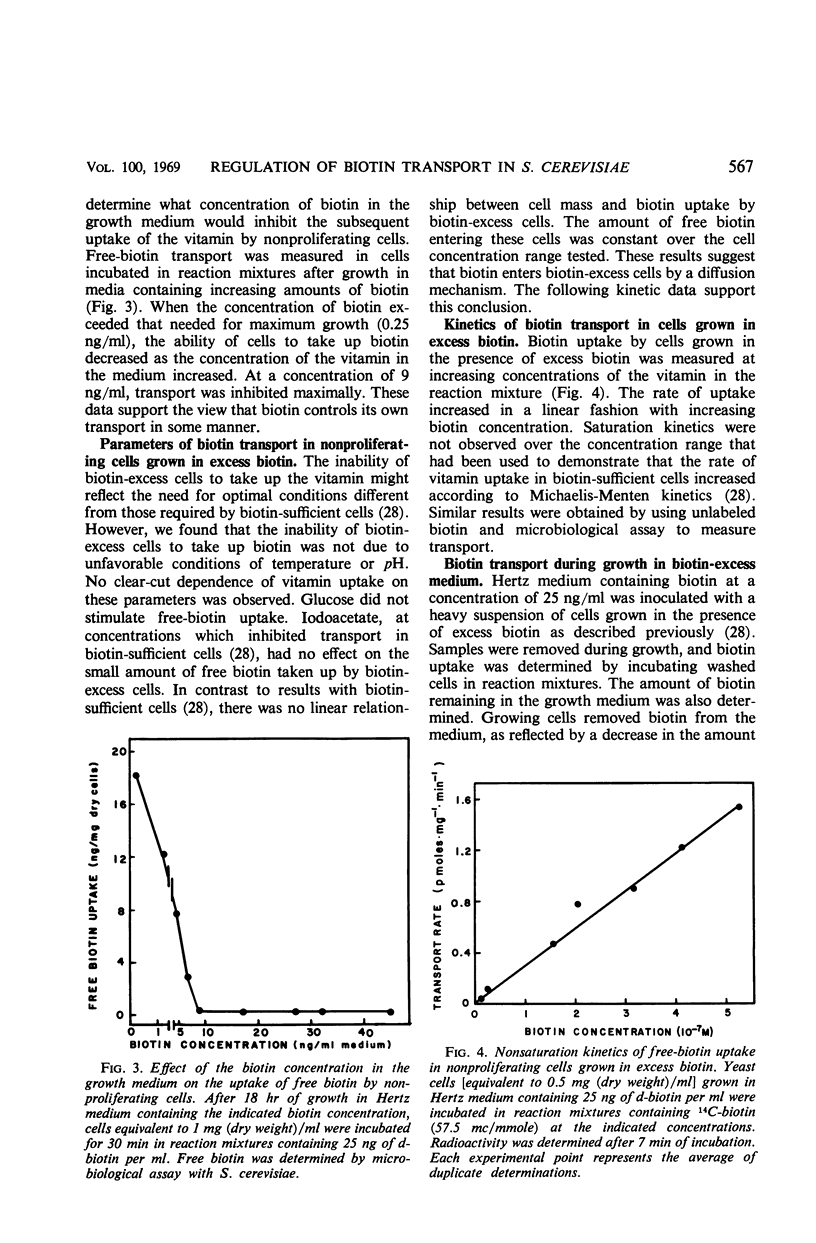
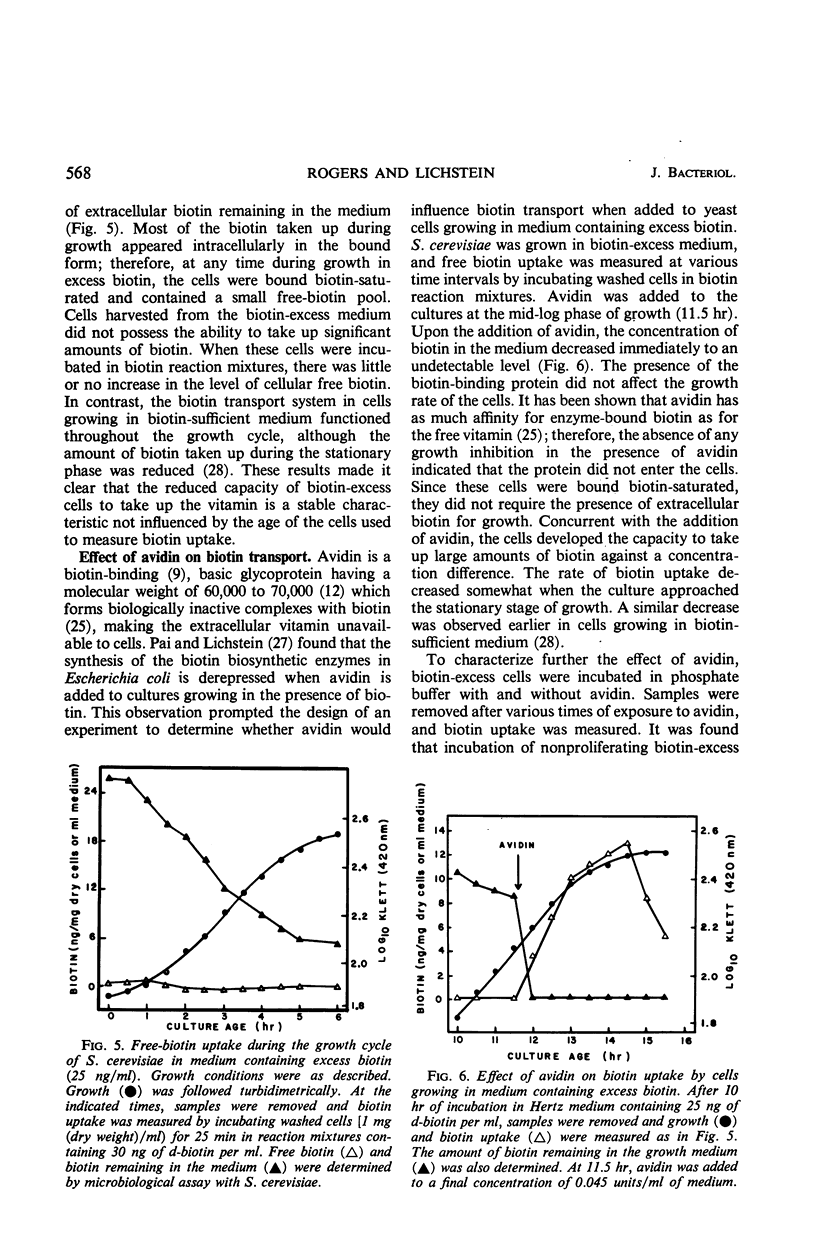
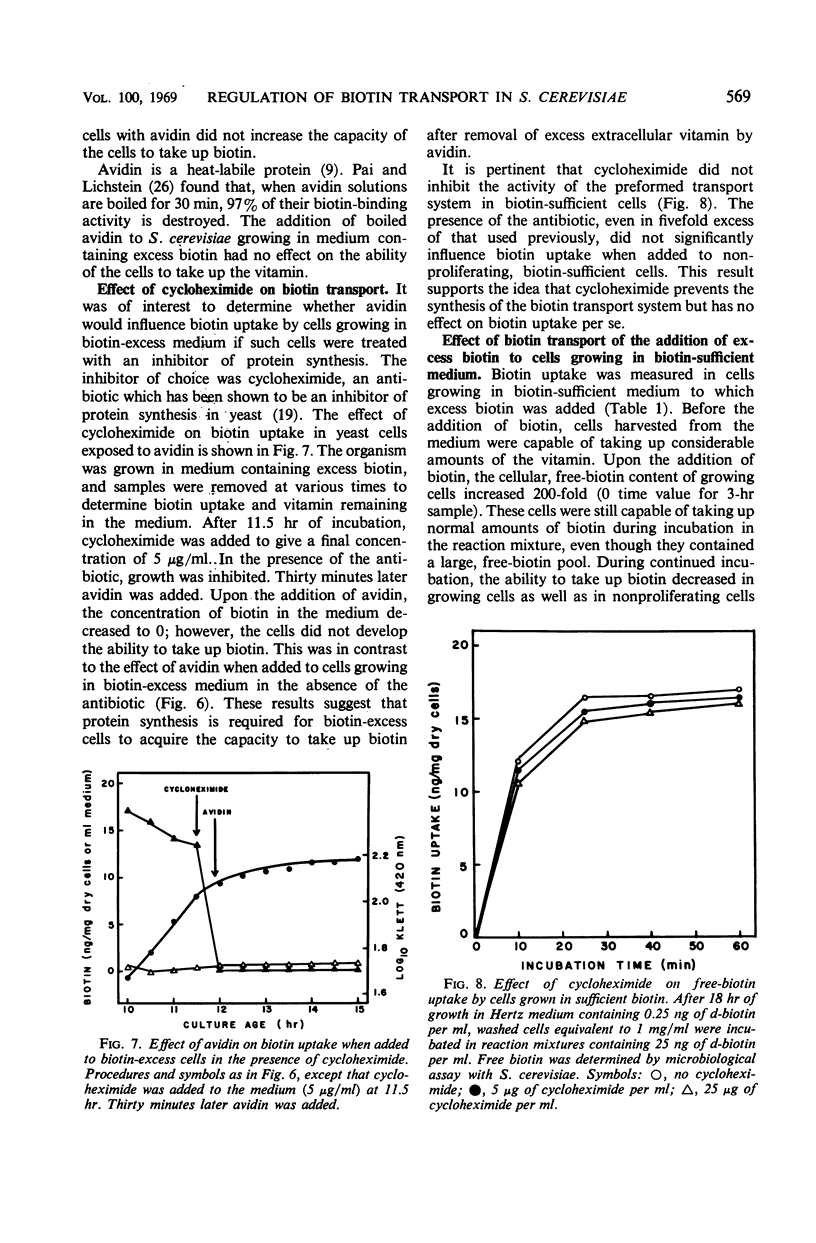
The metabolic control of biotin transport in Saccharomyces cerevisiae was investigated. Nonproliferating cells harvested from cultures grown in excess biotin (25 ng/ml) took up small amounts of biotin, whereas cells grown in biotin-sufficient medium (0.25 ng/ml) accumulated large amounts of the vitamin. Transport was inhibited maximally in cells grown in medium containing 9 ng (or more) of biotin per ml. When avidin was added to biotin-excess cultures, the cells developed the ability to take up large amounts of biotin. Boiled avidin was without effect, as was treatment of cells with avidin in buffer. Avidin did not relieve transport inhibition when added to biotin-excess cultures treated with cycloheximide, suggesting that protein synthesis was required for cells to develop the capacity to take up biotin after removal of extracellular vitamin by avidin. Cycloheximide did not inhibit the activity of the preformed transport system in biotin-sufficient cells. The presence of high intracellular free biotin pools did not inhibit the activity of the transport system. The characteristics of transport in biotin-excess cells (absence of temperature or pH dependence, no stimulation by glucose, absence of iodoacetate inhibition, independence of uptake on cell concentration, and nonsaturation kinetics) indicated that biotin entered these cells by diffusion. The results suggest that the synthesis of the biotin transport system in S. cerevisiae may be repressed during growth in medium containing high concentrations of biotin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959 Nov;78:601–612. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. S., Peterson W. H. FACTORS AFFECTING THE BIOTIN CONTENT OF YEASTS. J Bacteriol. 1949 Jul;58(1):33–44. doi: 10.1128/jb.58.1.33-44.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Regulation of sulfate transport in Salmonella typhimurium. J Bacteriol. 1966 Jun;91(6):2275–2280. doi: 10.1128/jb.91.6.2275-2280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- Goldman D., Schultz S. G., Epstein W. Repressive control of potassium transport in Escherichia coli. Biochim Biophys Acta. 1966 Dec 28;130(2):546–548. doi: 10.1016/0304-4165(66)90259-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Crabeel M., Wiame J. M., Béchet J. Inhibition of protein synthesis and simulation of permease turnover in yeast. Biochem Biophys Res Commun. 1968 Feb 26;30(4):414–419. doi: 10.1016/0006-291x(68)90760-2. [DOI] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- INUI Y., AKEDO H. AMINO ACID UPTAKE BY ESCHERICHIA COLI GROWN IN PRESENCE OF AMINO ACIDS. EVIDENCE FOR REPRESSIBILITY OF AMINO ACID UPTAKE. Biochim Biophys Acta. 1965 Jan 25;94:143–152. doi: 10.1016/0926-6585(65)90018-x. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of actidione and other antifungal agents on nucleic acid and protein synthesis in Saccharomyces carlsbergensis. J Gen Microbiol. 1958 Dec;19(3):497–506. doi: 10.1099/00221287-19-3-497. [DOI] [PubMed] [Google Scholar]
- KOGUT M., PODOSKI E. P. Oxidative pathways in a fluorescent Pseudomonas. Biochem J. 1953 Dec;55(5):800–811. doi: 10.1042/bj0550800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger K. K., Peterson W. H. Metabolism of Biotin and Oxybiotin by Lactobacillus pentosus 124-2. J Bacteriol. 1948 May;55(5):693–703. doi: 10.1128/jb.55.5.693-703.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHSTEIN H. C., WALLER J. R. Factors affecting the accumulation of biotin by Lactobacillus arabinosus. J Bacteriol. 1961 Jan;81:65–69. doi: 10.1128/jb.81.1.65-69.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon R. H., Rogers P., Hall W. H., Lichtein H. C. Inducible glutamate transport in Mycobacteria and its relation to glutamate oxidation. J Bacteriol. 1967 Jul;94(1):92–100. doi: 10.1128/jb.94.1.92-100.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACQUILLAN A. M., HALVORSON H. O. Physiological changes occurring in yeast undergoing glucose repression. J Bacteriol. 1962 Jul;84:31–36. doi: 10.1128/jb.84.1.31-36.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHOA S., KAZIRO Y. Biotin enzymes. Fed Proc. 1961 Dec;20:982–988. [PubMed] [Google Scholar]
- PAI C. H., LICHSTEIN H. C. OBSERVATIONS ON THE USE OF AVIDIN IN BACTERIOLOGICAL MEDIA. Proc Soc Exp Biol Med. 1964 May;116:197–200. doi: 10.3181/00379727-116-29200. [DOI] [PubMed] [Google Scholar]
- Pai C. H., Lichstein H. C. Biosynthesis of biotin in microorganisms. IV. Repression and derepression of (+ -)-biotin synthesis from (+)-desthiobiotin. Arch Biochem Biophys. 1966 Apr;114(1):138–144. doi: 10.1016/0003-9861(66)90314-6. [DOI] [PubMed] [Google Scholar]
- Rogers T. O., Lichstein H. C. Characterization of the biotin transport system in Saccharomyces cerevisiae. J Bacteriol. 1969 Nov;100(2):557–564. doi: 10.1128/jb.100.2.557-564.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHILO M., STANIER R. Y. The utilization of the tartaric acids by pseudomonads. J Gen Microbiol. 1957 Apr;16(2):482–490. doi: 10.1099/00221287-16-2-482. [DOI] [PubMed] [Google Scholar]
- Vogel H. J. REPRESSION OF AN ACETYLORNITHINE PERMEATION SYSTEM. Proc Natl Acad Sci U S A. 1960 Apr;46(4):488–494. doi: 10.1073/pnas.46.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. II. Metabolic control. J Bacteriol. 1968 Mar;95(3):959–966. doi: 10.1128/jb.95.3.959-966.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto L. A., Segel I. H. The inorganic sulfate transport system of Penicillium chrysogenum. Arch Biochem Biophys. 1966 Jun;114(3):523–538. doi: 10.1016/0003-9861(66)90376-6. [DOI] [PubMed] [Google Scholar]
- van Steveninck J., Dawson E. C. Active and passive galactose transport in yeast. Biochim Biophys Acta. 1968 Jan 3;150(1):47–55. doi: 10.1016/0005-2736(68)90007-2. [DOI] [PubMed] [Google Scholar]



