Abstract
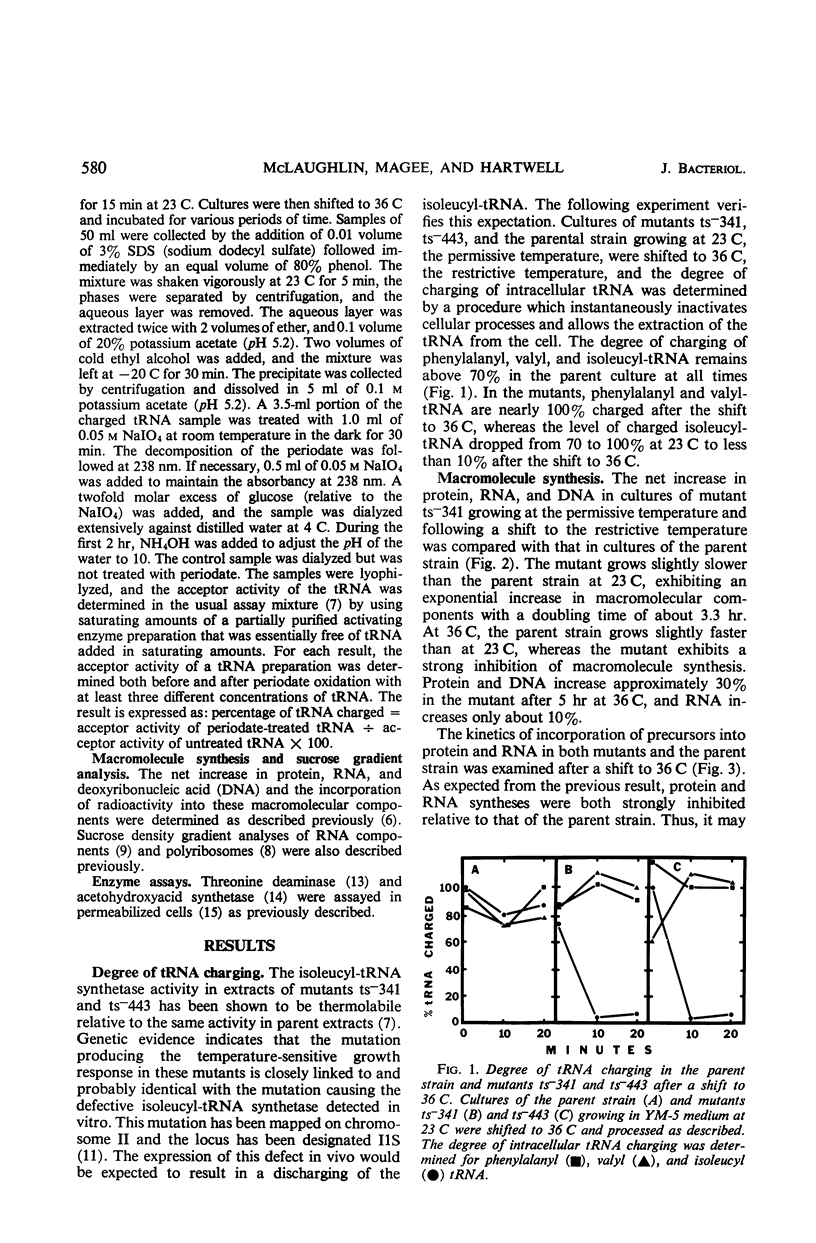
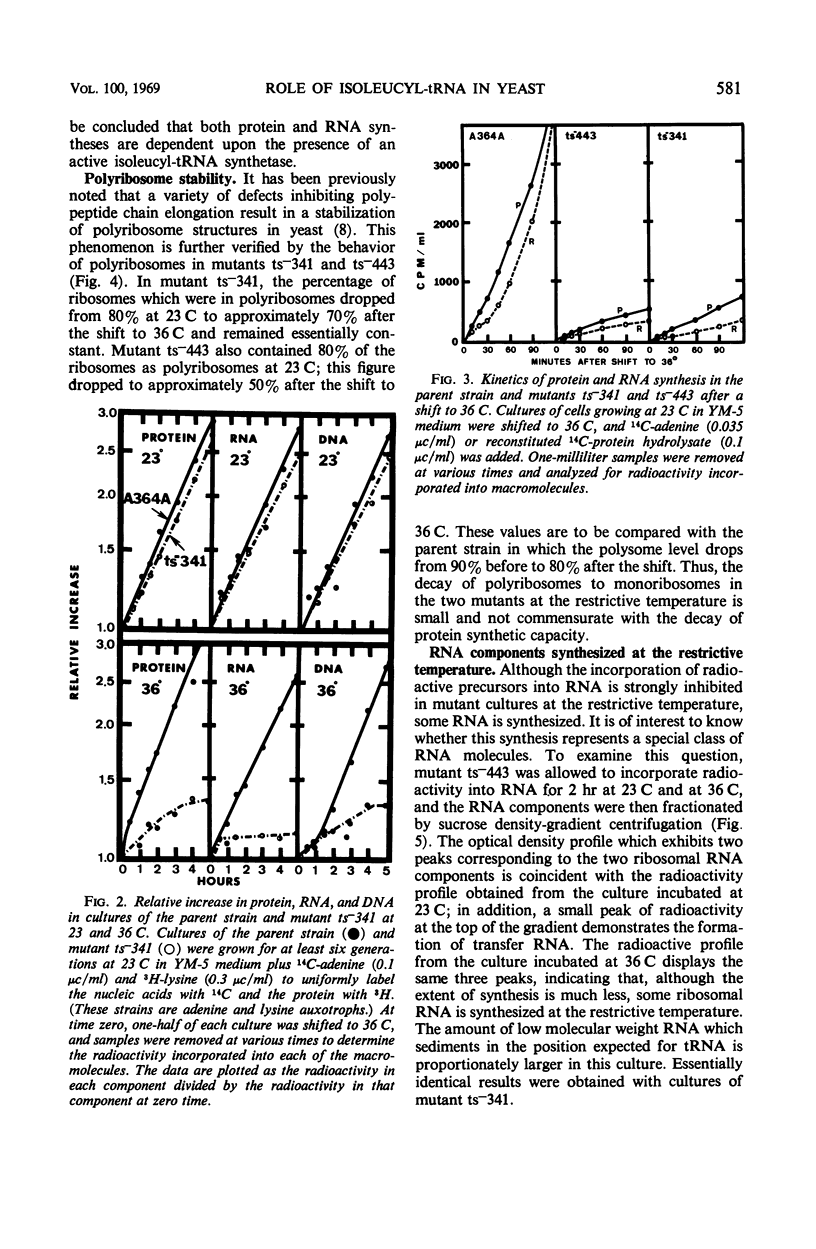
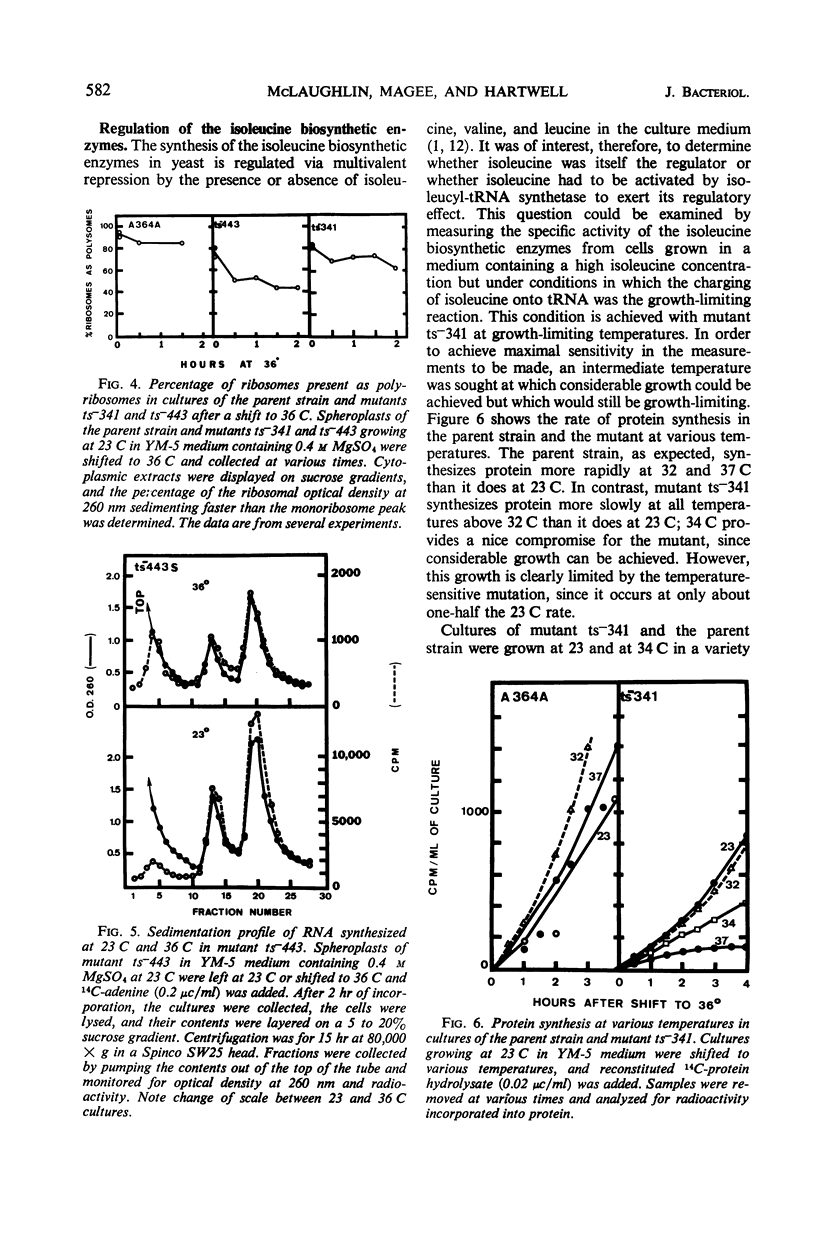
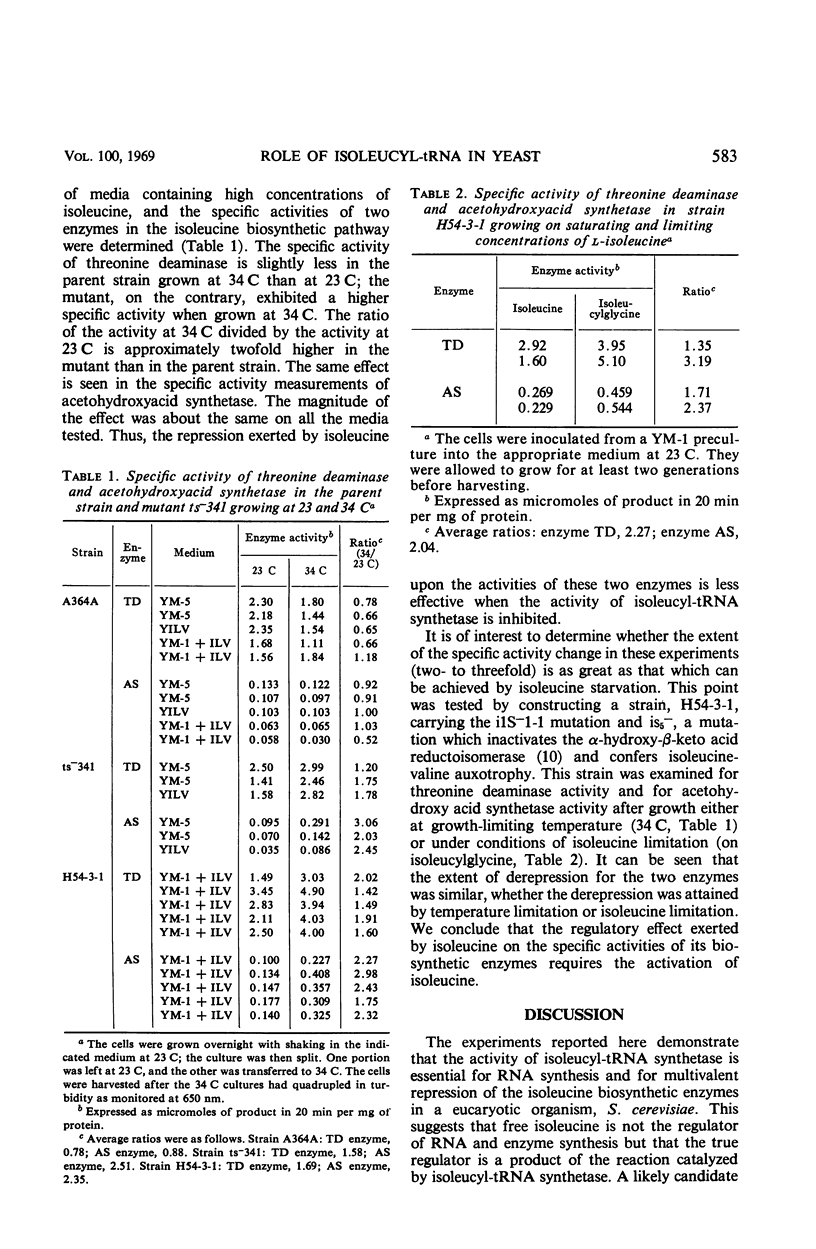
Temperature-sensitive mutations in the isoleucyl-transfer ribonucleic acid (tRNA) synthetase of yeast, ilS−1-1 and ilS−1-2, were used to examine the role of aminoacyl-tRNA synthetase enzymes in the regulation of ribonucleic acid (RNA) synthesis and enzyme synthesis in a eucaryotic organism. At the permissive temperature, 70 to 100% of the intracellular isoleucyl-tRNA was charged in mutants carrying these mutations; at growth-limiting temperatures, less than 10% was charged with isoleucine. Other aminoacyl-tRNA molecules remained essentially fully charged under both conditions. Net protein and RNA syntheses were rapidly inhibited when the mutant was shifted from the permissive to the restrictive temperature. Most of the ribosomes remained in polyribosome structures at the restrictive temperature even though protein synthesis was strongly inhibited. Two of the enzymes of isoleucine biosynthesis, threonine deaminase and acetohydroxyacid synthetase, were derepressed about twofold during slow growth of the mutants at a growth-limiting temperature. This is about the same degree of derepression that is achieved by growth of an auxotroph on limiting isoleucine. We conclude that charged aminoacyl-tRNA is essential for RNA synthesis and for the multivalent repression of the isoleucine biosynthetic enzymes. Aminoacyl tRNA synthetase enzymes appear to play important regulatory roles in the cell physiology of eucaryotic organisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bussey H., Umbarger H. E. Biosynthesis of branched-chain amino acids in yeast: regulation of synthesis of the enzymes of isoleucine and valine biosynthesis. J Bacteriol. 1969 May;98(2):623–628. doi: 10.1128/jb.98.2.623-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. PROTEIN AND RIBONUCLEIC ACID SYNTHESIS IN A MUTANT OF ESCHERICHIA COLI WITH AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE. J Biol Chem. 1964 Jun;239:1844–1847. [PubMed] [Google Scholar]
- Freundlich M. Valyl-Transfer RNA: Role in Repression of the Isoleucine-Valine Enzymes in Escherichia coli. Science. 1967 Aug 18;157(3790):823–825. doi: 10.1126/science.157.3790.823-a. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Mutants of yeast with temperature-sensitive isoleucyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1968 Feb;59(2):422–428. doi: 10.1073/pnas.59.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKAR S. N., WAGNER R. P. GENETIC AND BIOCHEMICAL ANALYSIS OF ISOLEUCINE-VALINE MUTANTS OF YEAST. Genetics. 1964 Feb;49:213–222. doi: 10.1093/genetics/49.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Hereford L. M. Multivalent repression of isoleucine- valine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jun;98(3):857–862. doi: 10.1128/jb.98.3.857-862.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 2. Identification and characterization of mutants lacking the acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):502–506. doi: 10.1111/j.1432-1033.1967.tb19559.x. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Hartwell L. H. A mutant of yeast with a defective methionyl-tRNA synthetase. Genetics. 1969 Mar;61(3):557–566. doi: 10.1093/genetics/61.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario M. The accumulation of argininosuccinate in Neurospora crassa. II. Inhibition of arginyl-tRNA synthesis by argininosuccinate. Biochim Biophys Acta. 1967 Aug 22;145(1):146–152. doi: 10.1016/0005-2787(67)90663-6. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Magee P. T. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. I. Threonine deaminase. Eur J Biochem. 1968 Feb;3(4):492–501. doi: 10.1111/j.1432-1033.1967.tb19558.x. [DOI] [PubMed] [Google Scholar]


