Abstract
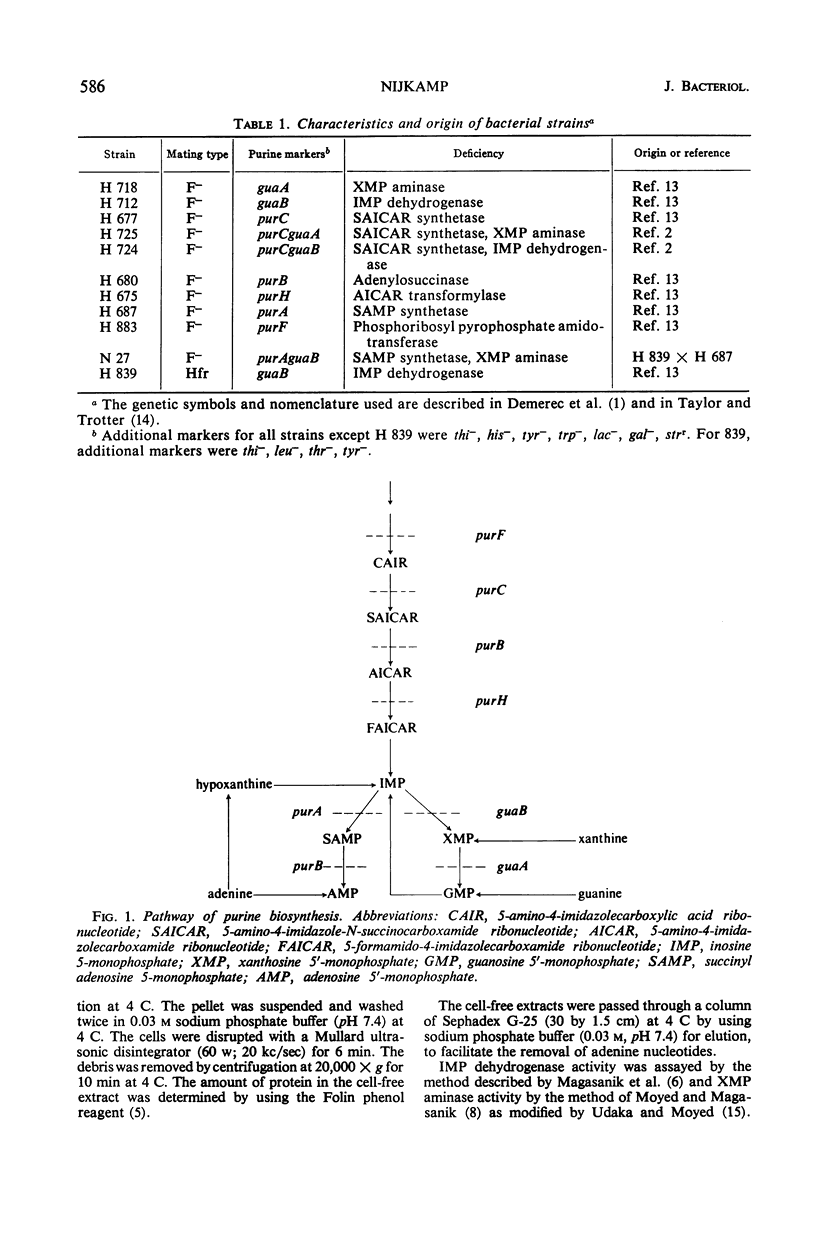
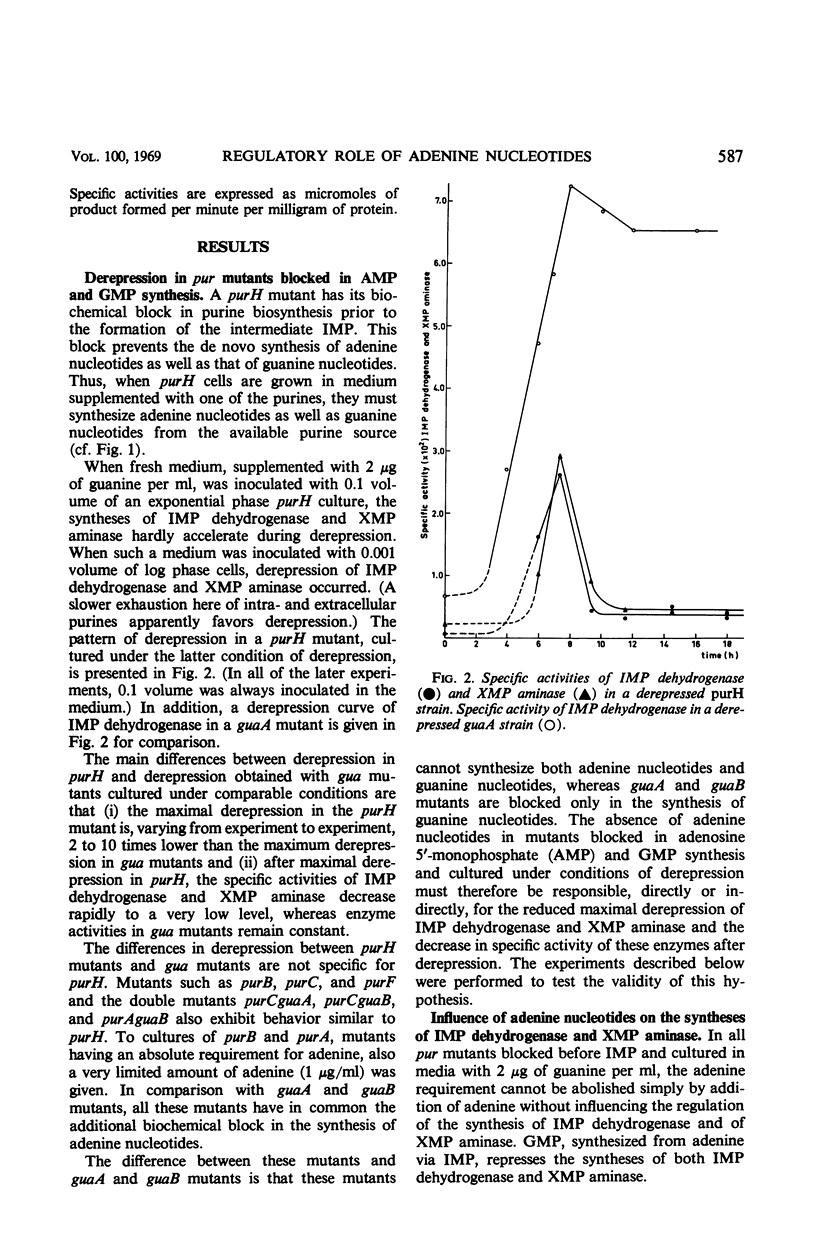
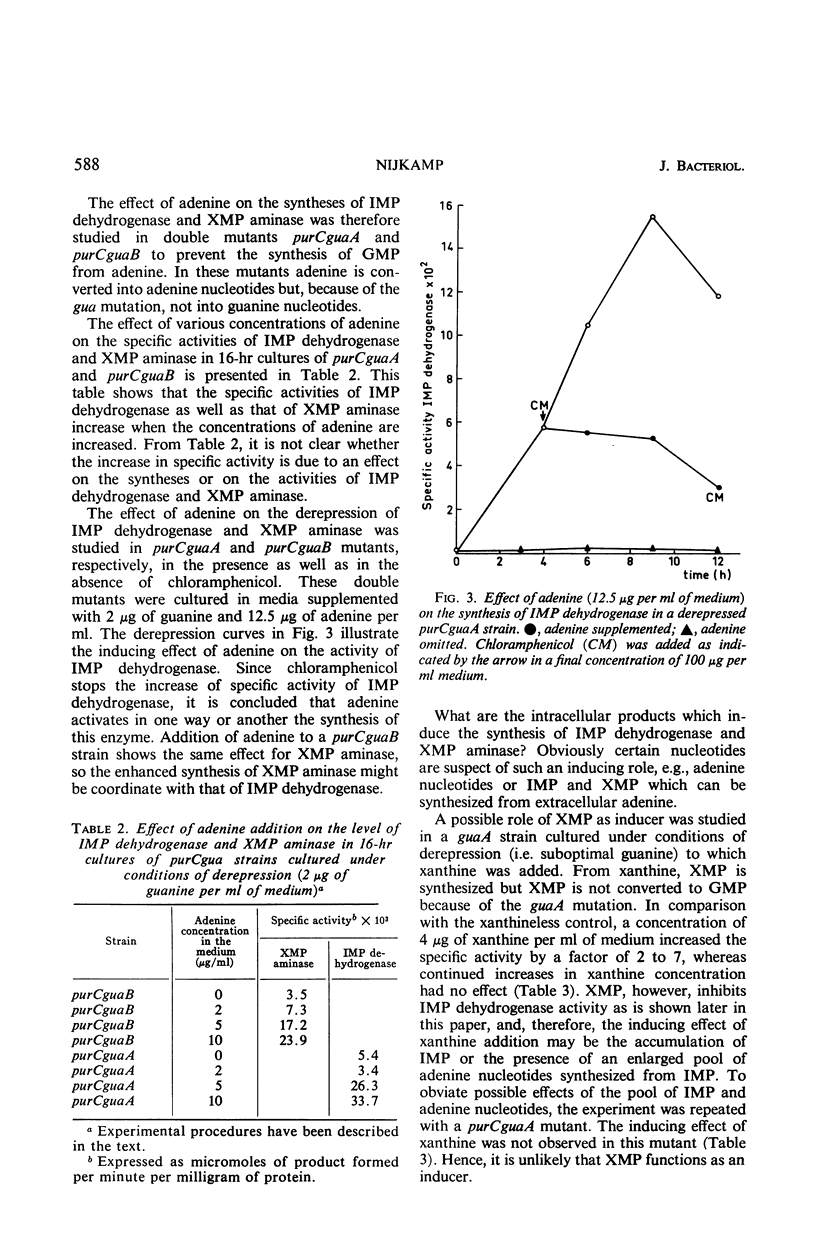
Derepression of the synthesis of inosine 5′-monophosphate (IMP) dehydrogenase and of xanthosine 5′-monophosphate (XMP) aminase in pur mutants of Escherichia coli which are blocked in the biosynthesis of adenine nucleotides and guanine nucleotides differs in two ways from derepression in pur mutants blocked exclusively in the biosynthesis of guanine nucleotides. (i) The maximal derepression is lower, and (ii) a sharp decrease in the specific activities of AMP dehydrogenase and XMP aminase occurs, following maximal derepression. From the in vivo and in vitro experiments described, it is shown that the lack of adenine nucleotides in derepressed pur mutants blocked in the biosynthesis of adenine and guanine nucleotides is responsible for these two phenomena. The adenine nucleotides are shown to play an important regulatory role in the biosynthesis of guanosine 5′-monophosphate (GMP). (i) They induce the syntheses of IMP dehydrogenase and XMP aminase. (The mechanism of induction may involve the expression of the gua operon.) (ii) They appear to have an activating function in IMP dehydrogenase and XMP aminase activity. The physiological importance of these regulatory characteristics of adenine nucleotides in the biosynthesis of GMP is discussed.
Full text
PDF
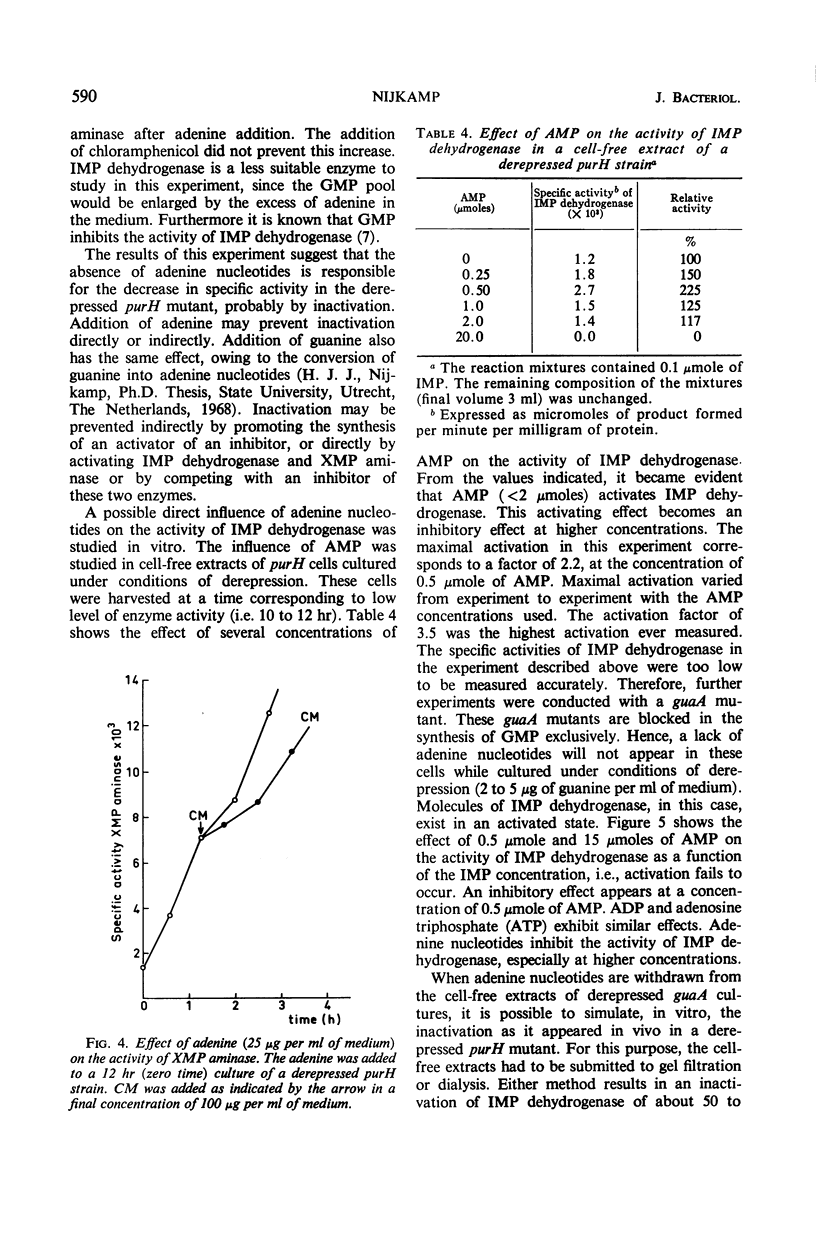
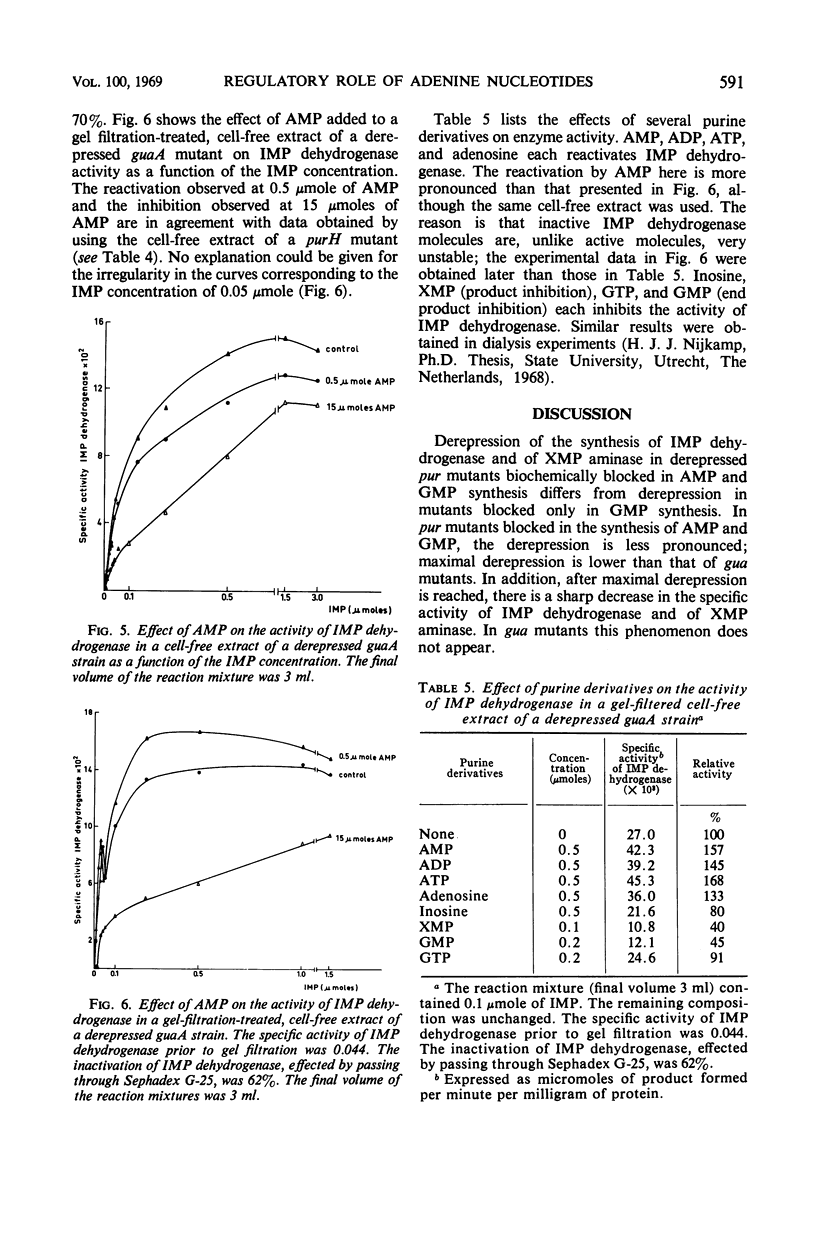


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUYAMA T., MOYED H. S. A SEPARATE ANTIBIOTIC-BINDING SITE IN XANTHOSINE-5'-PHOSPHATE AMINASE: INHIBITOR- AND SUBSTRATE-BINDING STUDIES. Biochemistry. 1964 Oct;3:1488–1492. doi: 10.1021/bi00898a017. [DOI] [PubMed] [Google Scholar]
- KURAMITSU H. K., UDAKA S., MOYED H. S. INDUCTION OF INOSINE 5'-PHOSPHATE DEHYDROGENASE AND XANTHOSINE 5'-PHOSPHATE AMINASE BY RIBOSYL-4-AMINO-5-IMIDAZOLECARBOXAMIDE IN PURINE-REQUIRING MUTANTS OF ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3425–3430. [PubMed] [Google Scholar]
- Kuramitsu H., Moyed H. S. A separate antibiotic binding site in xanthosine 5'-phosphate aminase. Differential alteration of catalytic properties and sensitivity to inhibition. J Biol Chem. 1966 Apr 10;241(7):1596–1601. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- MAGER J., MAGASANIK B. Guanosine 5'-phosphate reductase and its role in the interconversion of purine nucleotides. J Biol Chem. 1960 May;235:1474–1478. [PubMed] [Google Scholar]
- MOYED H. S., MAGASANIK B. Enzymes essential for the biosynthesis of nucleic acid guanine; xanthosine 5'-phosphate aminase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):351–363. [PubMed] [Google Scholar]
- NEUFELD E. F., KAPLAN N. O., COLOWICK S. P. Effect of adenine nucleotides on reactions involving triphosphopyridine nucleotide. Biochim Biophys Acta. 1955 Aug;17(4):525–535. doi: 10.1016/0006-3002(55)90415-7. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Oskamp A. A. Regulation of the biosynthesis of guanosine 5'-monophosphate: evidence for one operon. J Mol Biol. 1968 Jul 14;35(1):103–109. doi: 10.1016/s0022-2836(68)80040-3. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Stouthamer A. H., de Haan P. G., Nijkamp H. J. Mapping of purine markers in Escherichia coli K 12. Genet Res. 1965 Nov;6(3):442–453. doi: 10.1017/s0016672300004328. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDAKA S., MOYED H. S. INHIBITION OF PARENTAL AND MUTANT XANTHOSINE 5'-PHOSPHATE AMINASES BY PSICOFURANINE. J Biol Chem. 1963 Aug;238:2797–2803. [PubMed] [Google Scholar]
- Ullmann A., Monod J. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 1968 Nov;2(1):57–60. doi: 10.1016/0014-5793(68)80100-0. [DOI] [PubMed] [Google Scholar]


