Abstract
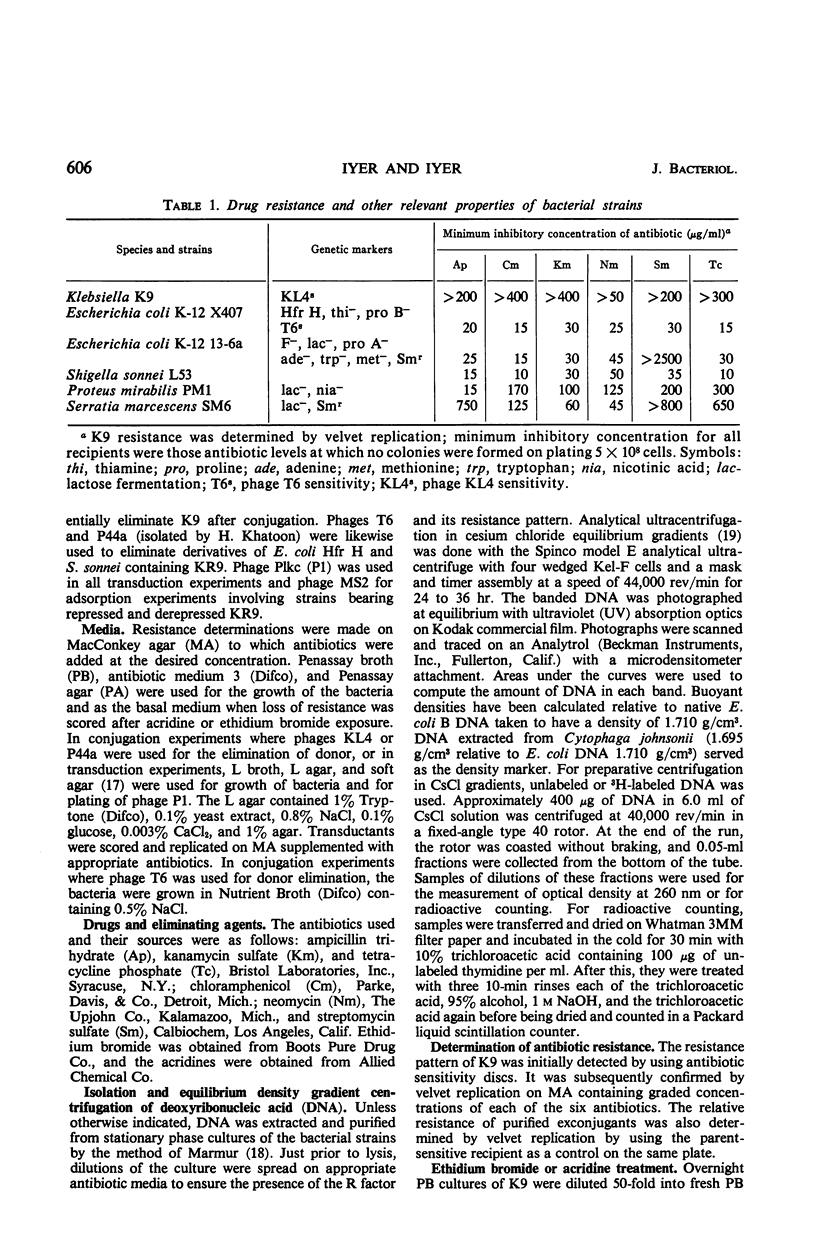
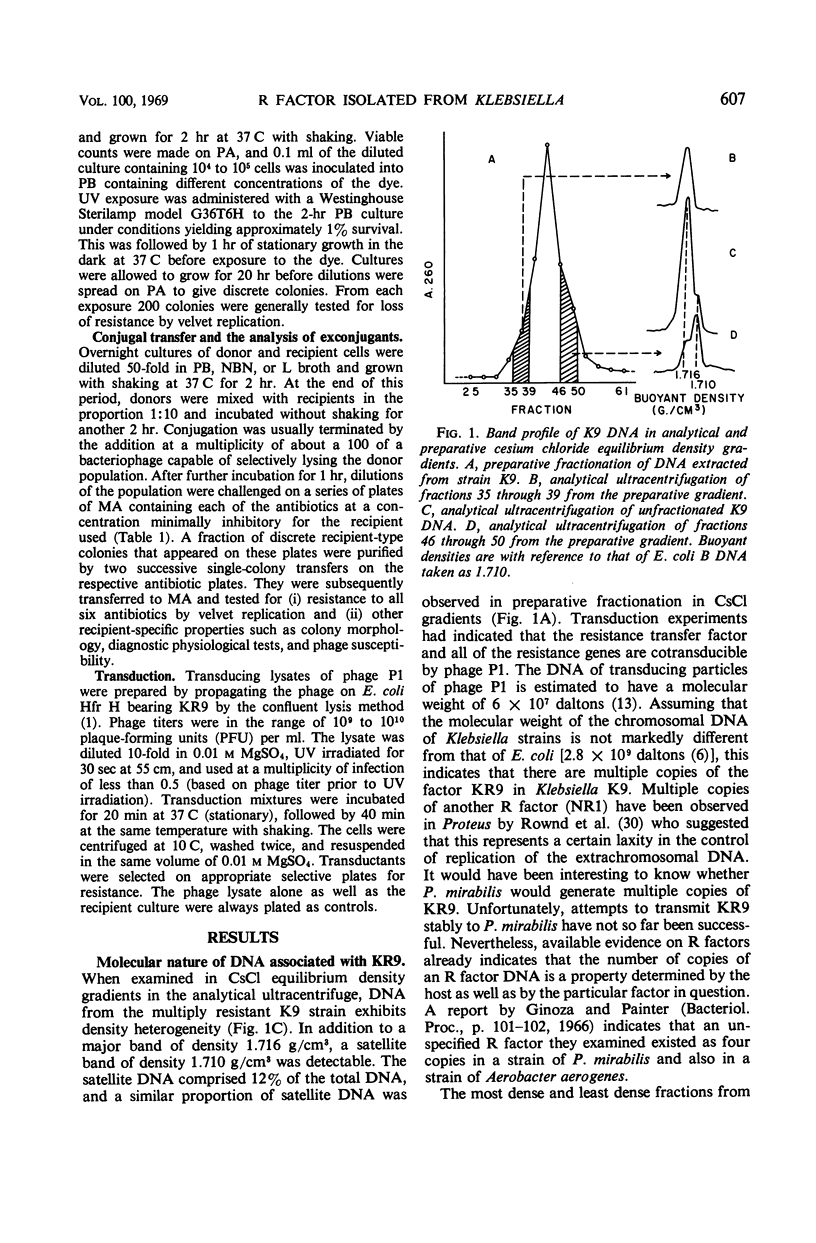
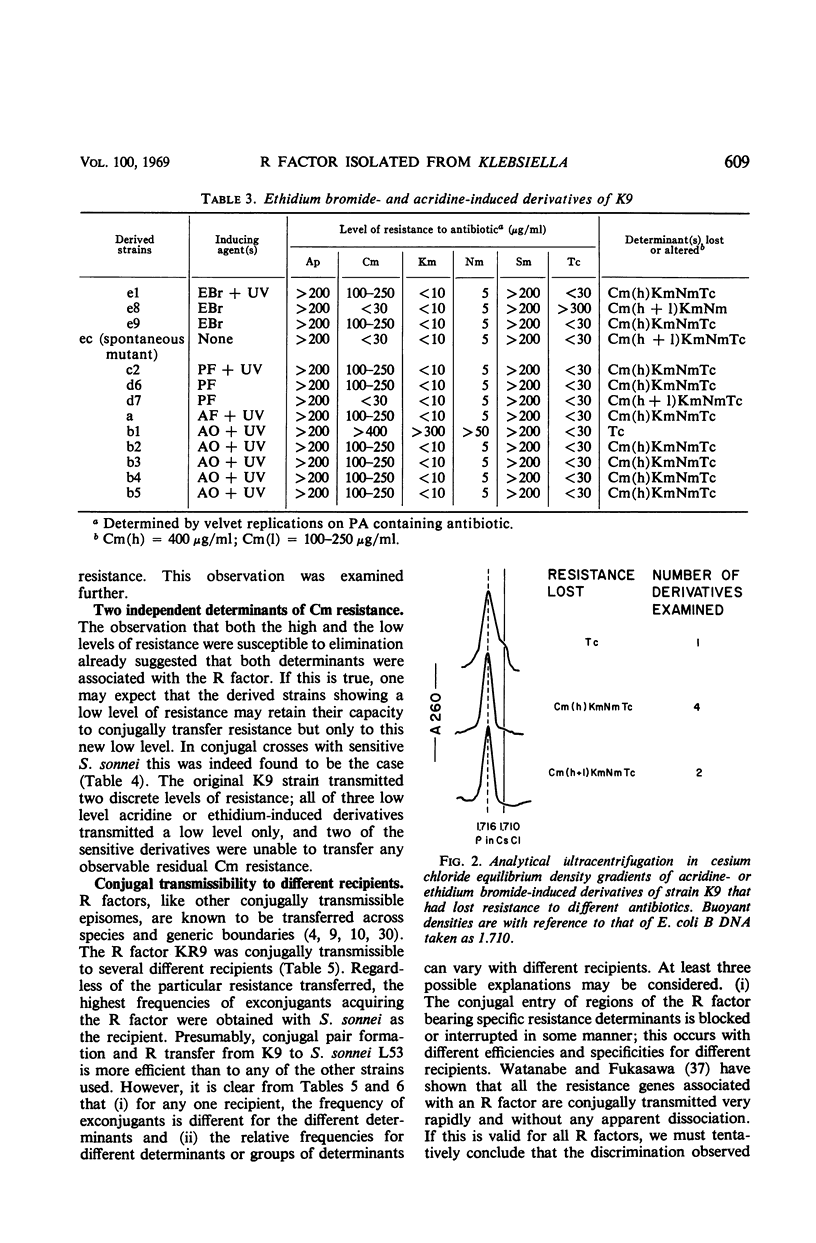
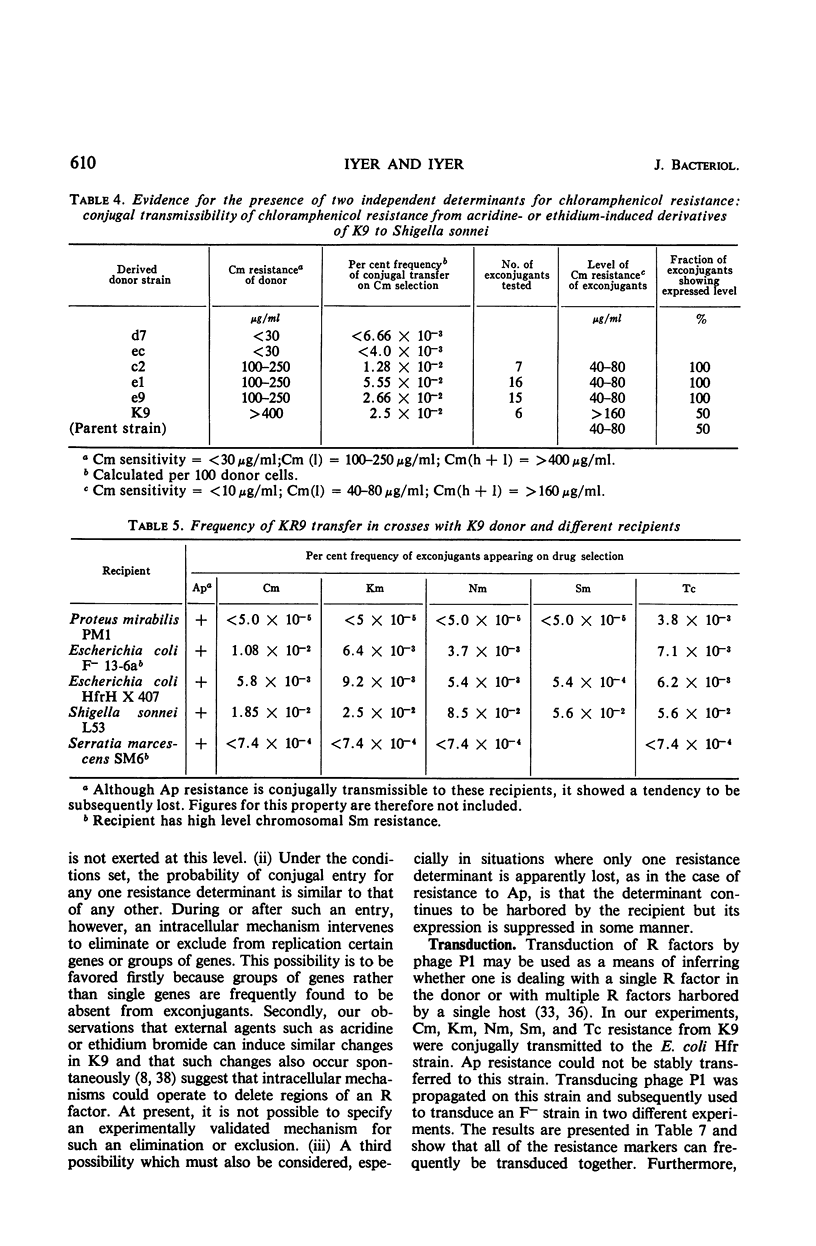
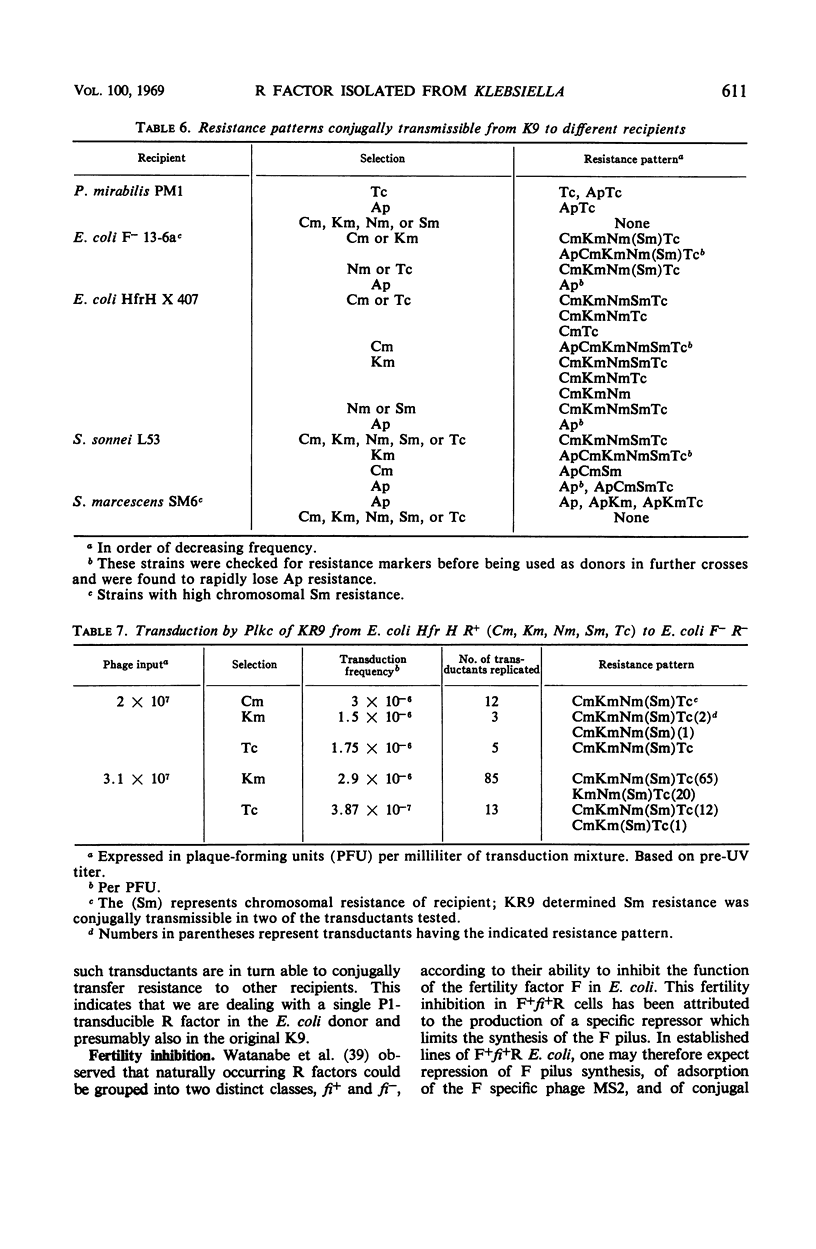
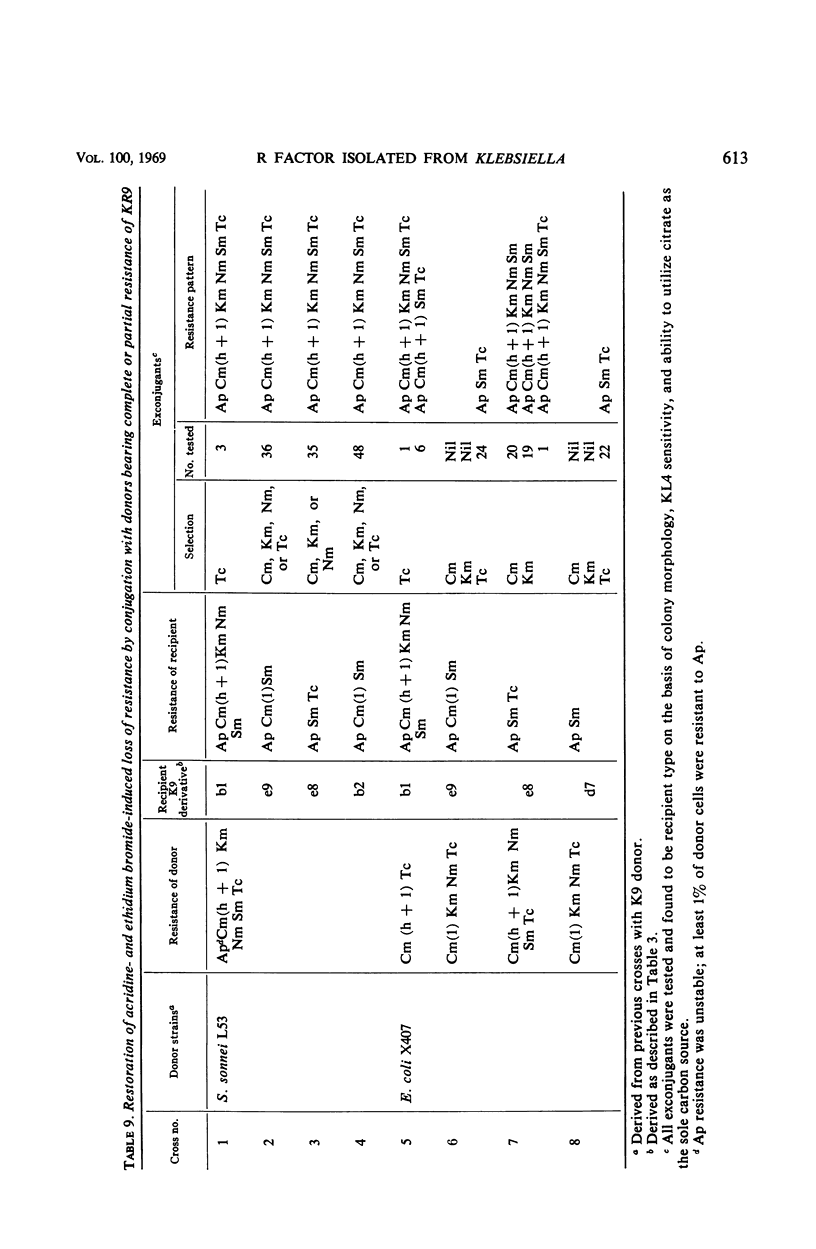
A Klebsiella strain of human origin that was resistant to ampicillin, chloramphenicol, kanamycin, neomycin, streptomycin, and tetracycline was found to have all of these resistances associated with a R factor and a satellite molecular species of deoxyribonucleic acid (DNA) with an average buoyant density of 1.710 in cesium chloride gradients. There was no evidence of the existence of DNA with other buoyant densities. The strain bears two separable mutations for chloramphenicol resistance, both of which are associated with the R factor (KR9). Exposure of the Klebsiella strain to acridine derivatives or to ethidium bromide (which was more efficient) resulted in partial losses of resistance accompanied by the disappearance of the satellite DNA peak or shifts in its density. The R factor and its component genes were conjugally transmitted across generic boundaries and maintained in new hosts with different efficiencies. The basis of this difference lies not only in the efficiency of conjugal transfer but also in the stability of the components after transfer. All of the resistance genes and the resistance transfer factor were cotransducible by phage Plkc from Escherichia coli. Partially resistant strains could be reconstituted to full resistance or to a recombined pattern of partial resistance by conjugation with donors having complementary resistance patterns. This recombination serves as an efficient mechanism for rescuing superinfecting genes that are otherwise intracellularly excluded. KR9 is an fi+ type of R factor which in the natural state does not appear to be as repressed in conjugal transfer as other R factors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Datta N., Lawn A. M., Meynell E. The relationship of F type piliation and F phage sensitivity to drug resistance transfer in R+F- Escherichia coli K 12. J Gen Microbiol. 1966 Nov;45(2):365–376. doi: 10.1099/00221287-45-2-365. [DOI] [PubMed] [Google Scholar]
- Falkow S., Citarella R. V., Wohlhieter J. A. The molecular nature of R-factors. J Mol Biol. 1966 May;17(1):102–116. doi: 10.1016/s0022-2836(66)80097-9. [DOI] [PubMed] [Google Scholar]
- GINOZA H. S., MATNEY T. S. TRANSMISSION OF A RESISTANCE TRANSFER FACTOR FROM ESCHERICHIA COLI TO 2 SPECIES OF PASTEURELLA. J Bacteriol. 1963 May;85:1177–1178. doi: 10.1128/jb.85.5.1177-1178.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARADA K., SUZUKI M., KAMEDA M., MITSUHASHI S. On the drug-resistance of enteric bacteria. 2) Transmission of the drug-resistance among Enterobacteriaceae. Jpn J Exp Med. 1960 Aug;30:289–299. [PubMed] [Google Scholar]
- HIROTA Y., LIJIMA T. Acriflavine as an effective agent for eliminating F-factor in Escherichia coli K-12. Nature. 1957 Sep 28;180(4587):655–656. doi: 10.1038/180655a0. [DOI] [PubMed] [Google Scholar]
- Hinshaw V., Punch J., Allison M. J., Dalton H. P. Frequency of R factor-mediated multiple drug resistance in Klebsiella and Aerobacter. Appl Microbiol. 1969 Feb;17(2):214–218. doi: 10.1128/am.17.2.214-218.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Kontomichalou P. Studies on resistance transfer factors. Pathol Microbiol (Basel) 1967;30(1):71–93. doi: 10.1159/000161646. [DOI] [PubMed] [Google Scholar]
- Kétyi I., Vertényi A. Segregation of R factor in Shigella after acridine orange treatment. Acta Microbiol Acad Sci Hung. 1967;14(2):181–188. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HARADA K., HASHIMOTO H., KAMEDA M., SUZUKI M. Combination of two types of transmissible drug-resistance factors in a host bacterium. J Bacteriol. 1962 Jul;84:9–16. doi: 10.1128/jb.84.1.9-16.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Hashimoto H., Egawa R., Tanaka T., Nagai Y. Drug resistance of enteric bacteria. IX. Distribution of R factors in gram-negative bacteria from clinical sources. J Bacteriol. 1967 Apr;93(4):1242–1245. doi: 10.1128/jb.93.4.1242-1245.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Doherty P. Linkage relationships of two genes causing partial resistance to chloramphenicol in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1450–1451. doi: 10.1128/jb.96.4.1450-1451.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E., Meynell E. Covert fi- R factors in fi+ R+ strains of bacteria. J Bacteriol. 1969 Feb;97(2):780–786. doi: 10.1128/jb.97.2.780-786.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Salzman T. C., Klemm L. Transferable drug resistance (R factors) in Enterobacteriaceae: relationship to nosocomial infections. Antimicrob Agents Chemother (Bethesda) 1966;6:212–220. [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- WATANABE T. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VI. HIGH-FREQUENCY RESISTANCE TRANSFER SYSTEM IN ESCHERICHIA COLI. J Bacteriol. 1963 Apr;85:788–794. doi: 10.1128/jb.85.4.788-794.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. III. Transduotion of resistance factors. J Bacteriol. 1961 Aug;82:202–209. doi: 10.1128/jb.82.2.202-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., LYANG K. W. Episome-mediated transfer of drug resistance in Enterobacteriaceae. V. Spontaneous segregation and recombination of resistance factors in Salmonella typhimurium. J Bacteriol. 1962 Sep;84:422–430. doi: 10.1128/jb.84.3.422-430.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Sakaizumi S., Furuse C. Superinfection with R factors by transduction in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1796–1802. doi: 10.1128/jb.96.5.1796-1802.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



