Abstract
The cellular morphology of a biochemical variant of Candida albicans could be controlled by the ratio of carbon dioxide to oxygen in the culture system or by individual amino acids. Predominantly pseudohyphal morphology was observed (i) at a CO2 to O2 ratio of 2:1 and (ii) without the addition of carbon dioxide, when either glycine, d- or l-ornithine, l-serine, l-methionine, l-phenylalanine, or l-tyrosine was the sole nitrogen source in the culture medium. When ammonium chloride, ammonium sulfate, l-glutamic acid, l-glutamine, or l-proline was the nitrogen source, yeastlike growth was observed in the presence or absence of CO2. More adenosylmethionine was present in pseudohyphal than in yeastlike cells, and pseudohyphal cell wall preparations contained less methionine than cell walls from the yeastlike form. These results suggest a correlation between sulfur amino acid metabolism and dimorphism.
Full text
PDF

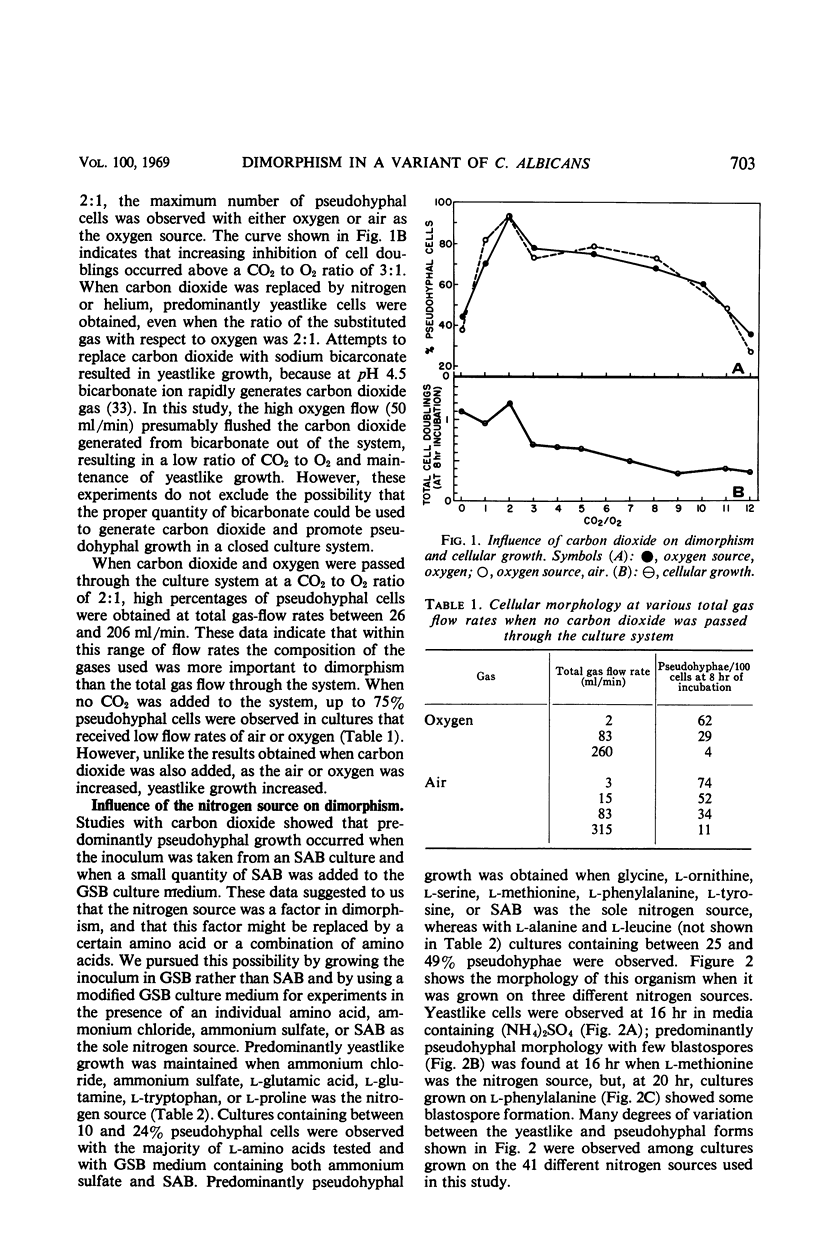
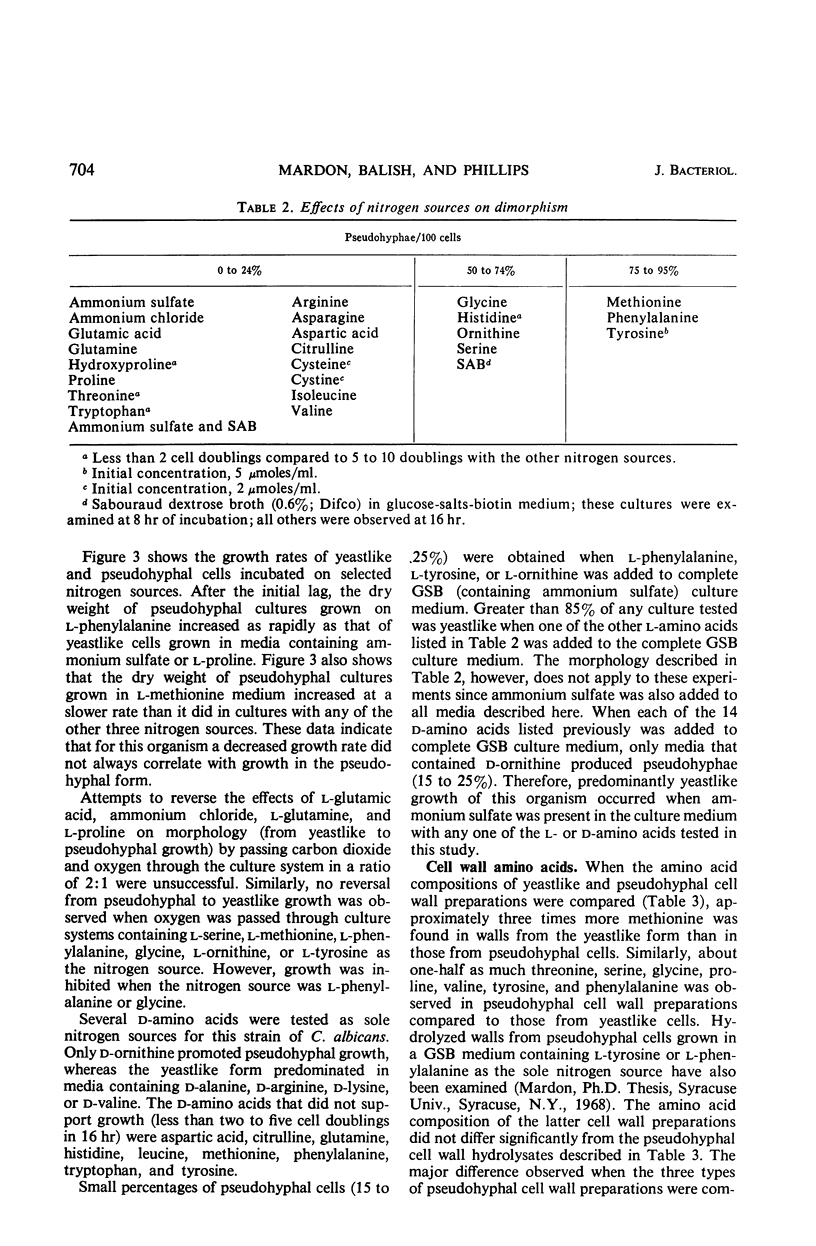



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI GARCIA S. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. III. MOLD-YEAST DIMORPHISM OF MUCOR. Bacteriol Rev. 1963 Sep;27:293–304. doi: 10.1128/br.27.3.293-304.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS L. D., NORTHEY W. T. Studies on coccidioides immitis. II. Physiological studies on in vitro spherulation. J Bacteriol. 1963 Jan;85:12–15. doi: 10.1128/jb.85.1.12-15.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Svihla G. Ultraviolet microscopy of Candida albicans. J Bacteriol. 1966 Dec;92(6):1812–1820. doi: 10.1128/jb.92.6.1812-1820.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Control of dimorphism in Mucor by hexoses: inhibition of hyphal morphogenesis. J Bacteriol. 1968 Nov;96(5):1586–1594. doi: 10.1128/jb.96.5.1586-1594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Thomsen J. Reverse variations between Candida albicans and Candida tropicalis? Acta Pathol Microbiol Scand. 1966;66(1):143–144. doi: 10.1111/apm.1966.66.1.143. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Hough J. S. Elongation of yeast cells in continuous culture. Nature. 1965 May 15;206(985):676–678. doi: 10.1038/206676a0. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- FALCONE G., NICKERSON W. J. Identification of protein disulfide reductase as a cellular division enzyme in yeasts. Science. 1956 Oct 19;124(3225):722–723. doi: 10.1126/science.124.3225.722. [DOI] [PubMed] [Google Scholar]
- GILARDI G. L., LAFFER N. C. Nutritional studies on the yeast phase of Blastomyces dermatitidis and B. brasiliensis. J Bacteriol. 1962 Feb;83:219–227. doi: 10.1128/jb.83.2.219-227.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Nutrition of systemic and subcutaneous pathogenic fungi. Bacteriol Rev. 1965 Sep;29(3):406–424. doi: 10.1128/br.29.3.406-424.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER G., NICKERSON W. J. Glucomannan-protein complexes from cell walls of yeasts. J Biol Chem. 1959 Sep;234:2281–2285. [PubMed] [Google Scholar]
- LONES G. W., PEACOCK C. L. Role of carbon dioxide in the dimorphism of Coccidioides immitis. J Bacteriol. 1960 Feb;79:308–309. doi: 10.1128/jb.79.2.308-309.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORDEN A. Sporotrichosis; clinical and laboratory features and a serologic study in experimental animals and humans. Acta Pathol Microbiol Scand Suppl. 1951;89:1–119. [PubMed] [Google Scholar]
- SCHLENK F., EHNINGER D. J. OBSERVATIONS ON THE METABOLISM OF 5'-METHYLTHIOADENOSINE. Arch Biochem Biophys. 1964 Jul 20;106:95–100. doi: 10.1016/0003-9861(64)90161-4. [DOI] [PubMed] [Google Scholar]
- SENTHESHANMUGANATHAN S., NICKERSON W. J. Nutritional control of cellular form in Trigonopsis variabilis. J Gen Microbiol. 1962 Mar;27:437–449. doi: 10.1099/00221287-27-3-437. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Sundhagul M., Hedrick L. R. Effect of tryptophan on growth and morphology of Hansenula schneggii cells. J Bacteriol. 1966 Jul;92(1):241–249. doi: 10.1128/jb.92.1.241-249.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Stock J. J. Biochemical Alterations of Dermatophytes during Growth. Appl Microbiol. 1966 May;14(3):438–444. doi: 10.1128/am.14.3.438-444.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]




