Abstract
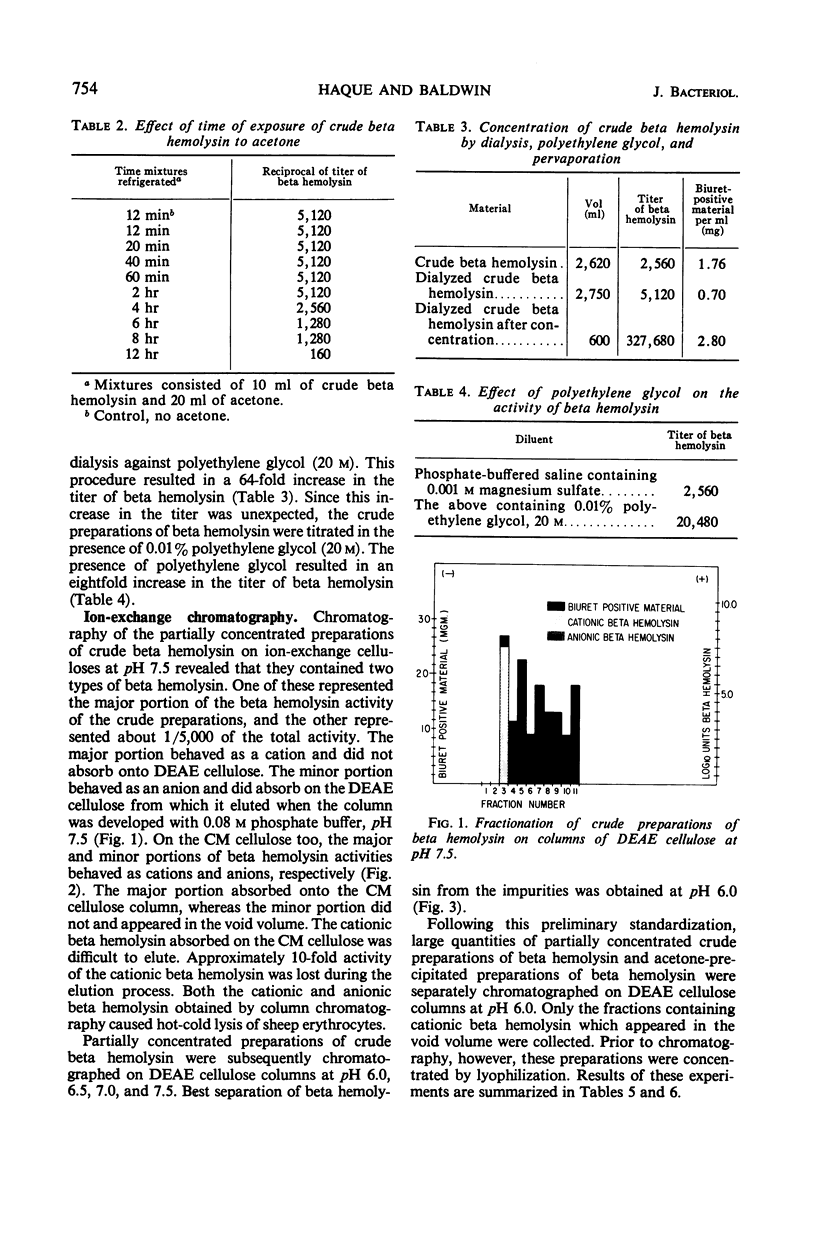
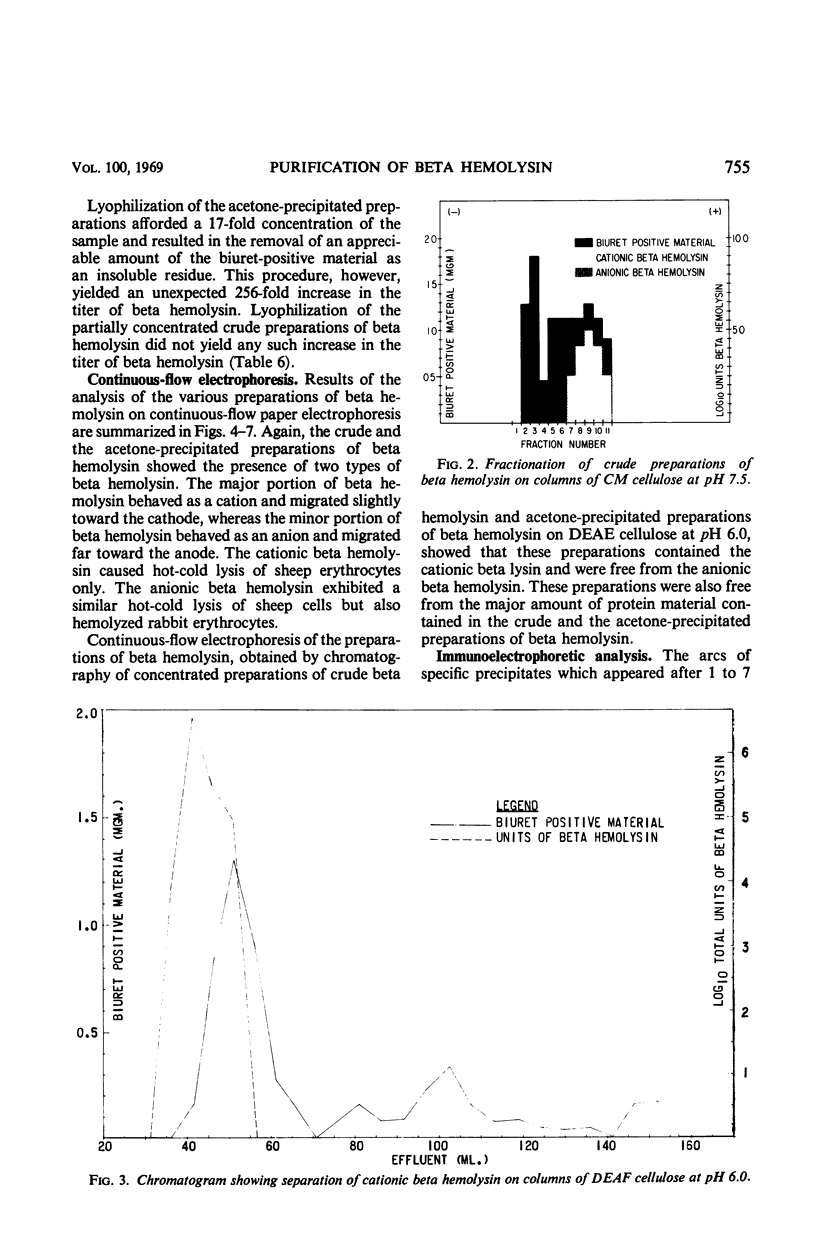
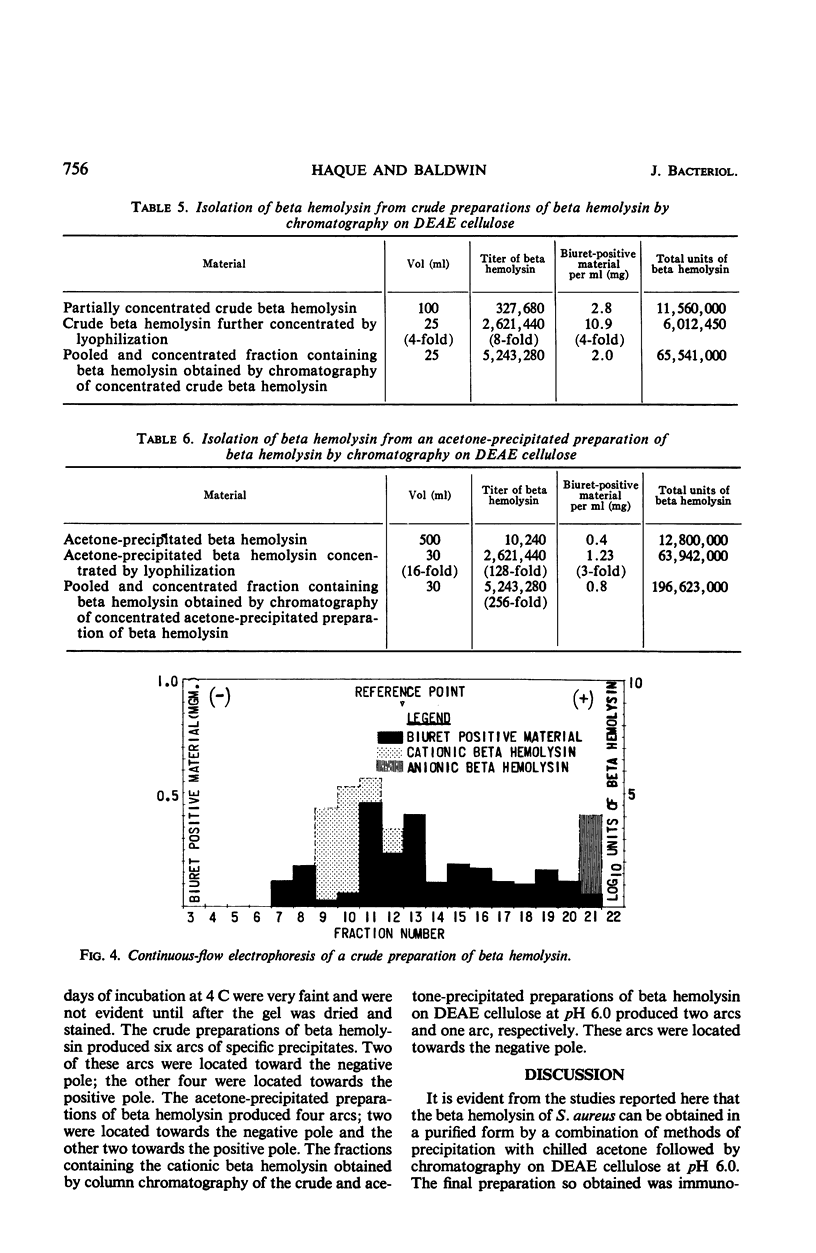
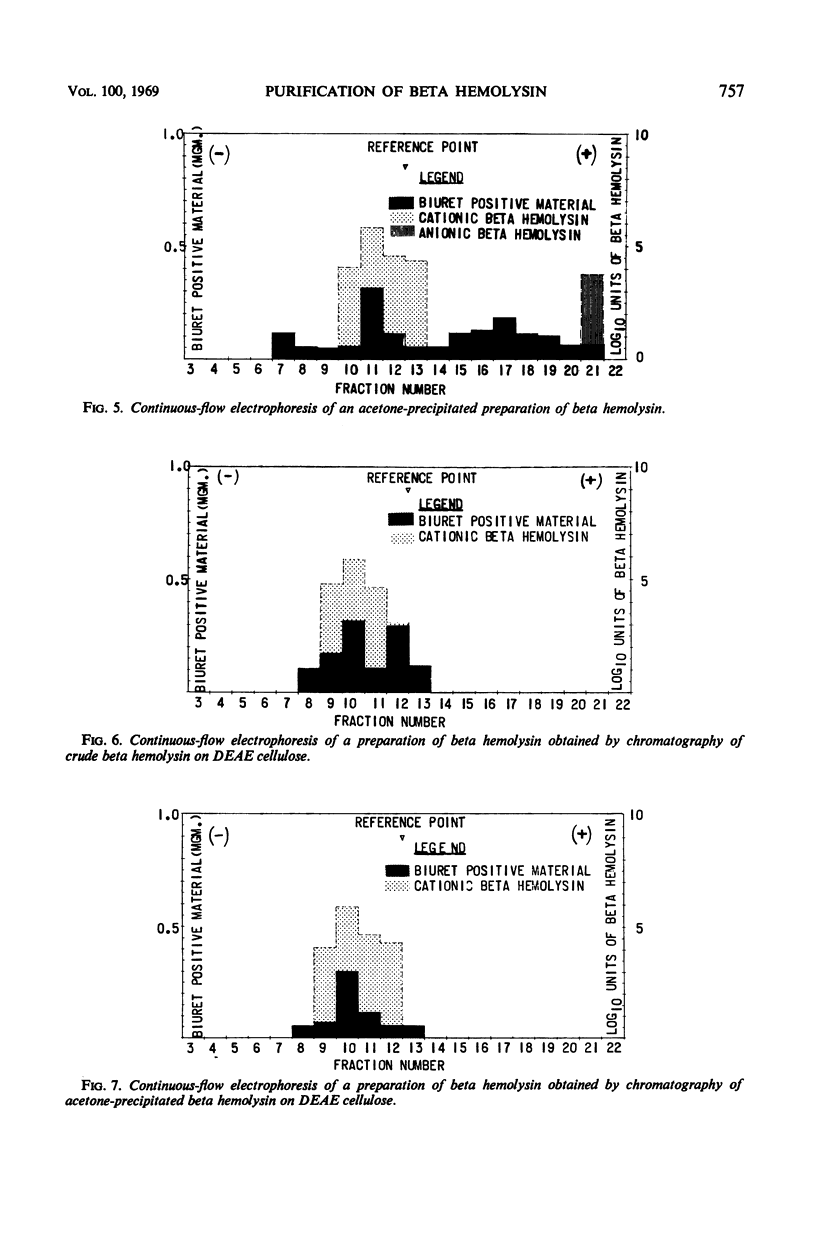
Staphylococcal beta hemolysin from the 681 strain of Staphylococcus aureus grown in a Heart Infusion dialysate semisolid medium under 10% carbon dioxide was obtained in an immunoelectrophoretically pure form by a combination of procedures of precipitation with 2 volumes of acetone followed by chromatography on diethylaminoethyl cellulose at pH 6.0. The acetone precipitation procedure did not show any deleterious effect on the hemolytic activity of the beta hemolysin unless the precipitate was left in contact with the acetone for at least 4 hr. The crude preparations contained two types of beta hemolysin. One of these represented the major portion of the total activity of beta hemolysin and behaved as a cation. The other represented a minor (1/5,000) portion of the total beta hemolysin activity and behaved as an anion. These active principles were designated as cationic and anionic beta hemolysins, respectively. An unexpected increase in the total beta hemolysin activity of the crude preparations was noted when these were concentrated by dialysis against polyethylene glycol (20 m). This effect was probably due to polyethylene glycol. A further unexpected increase in the titer of the acetone-precipitated preparations occurred when these were lyophilized. The reason for this incremental increase is not known. It may be due to fragmentation of the beta hemolysin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLANCO A. [The application of continuous flow electrophoresis to the study of the enzymatic activity of protein fractions of the blood serum]. Rev Fac Cienc Med Cordoba. 1961 Jul-Sep;19:149–158. [PubMed] [Google Scholar]
- CHESBRO W. R., HEYDRICK F. P., MARTINEAU R., PERKINS G. N. PURIFICATION OF STAPHYLOCOCCAL BETA-HEMOLYSIN AND ITS ACTION ON STAPHYLOCOCCAL AND STREPTOCOCCAL CELL WALLS. J Bacteriol. 1965 Feb;89:378–389. doi: 10.1128/jb.89.2.378-389.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doery H. M., Magnusson B. J., Gulasekharam J., Pearson J. E. The properties of phospholipase enzymes in staphylococcal toxins. J Gen Microbiol. 1965 Aug;40(2):283–296. doi: 10.1099/00221287-40-2-283. [DOI] [PubMed] [Google Scholar]
- Gow J. A., Robinson J. Properties of purified staphylococcal beta-hemolysin. J Bacteriol. 1969 Mar;97(3):1026–1032. doi: 10.1128/jb.97.3.1026-1032.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAQUE R. U., BALDWIN J. N. PURIFICATION AND PROPERTIES OF STAPHYLOCOCCAL BETA-HEMOLYSIN. I. PRODUCTION OF BETA-HEMOLYSIN. J Bacteriol. 1964 Nov;88:1304–1309. doi: 10.1128/jb.88.5.1304-1309.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R. U. Identification of staphylococcal hemolysins by an electrophoretic localization technique. J Bacteriol. 1967 Feb;93(2):525–530. doi: 10.1128/jb.93.2.525-530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S. K., Lindorfer R. K. Staphylococcal beta-hemolysin. II. Phospholipase C activity of purified beta-hemolysin. J Bacteriol. 1967 Nov;94(5):1313–1319. doi: 10.1128/jb.94.5.1313-1319.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S. K., Smith K. L., Lindorfer R. K. Saphylococcal beta-hemolysin. I. Purification of beta-hemolysin. J Bacteriol. 1967 Aug;94(2):300–305. doi: 10.1128/jb.94.2.300-305.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


