Abstract
Two isoforms of human interleukin 15 (IL-15) exist. One isoform has a shorter putative signal peptide (21 amino acids) and its transcript shows a tissue distribution pattern that is distinct from that of the alternative IL-15 isoform with a 48-aa signal peptide. The 21-aa signal isoform is preferentially expressed in tissues such as testis and thymus. Experiments using different combinations of signal peptides and mature proteins (IL-2, IL-15, and green fluorescent protein) showed that the short signal peptide regulates the fate of the mature protein by controlling the intracellular trafficking to nonendoplasmic reticulum sites, whereas the long signal peptide both regulates the rate of protein translation and functions as a secretory signal peptide. As a consequence, the IL-15 associated with the short signal peptide is not secreted, but rather is stored intracellularly, appearing in the nucleus and cytoplasmic components. Such production of an intracellular lymphokine is not typical of other soluble interleukin systems, suggesting a biological function for IL-15 as an intracellular molecule.
Keywords: splice variant, posttranscriptional control
Interleukin 15 (IL-15) was identified as a T cell growth factor (1–3). Similar to other interleukins sharing this property, IL-15 functions through the common γ receptor in T and NK cells (1, 3, 4). In addition, IL-15 and IL-2 share the β chain of the receptor in these cells (1, 4) and consequently share many biological properties, including T cell- and NK cell-growth promoting activity. However, unlike IL-2, IL-15 mRNA is expressed in many nonlymphoid cells, including monocytes, fibroblasts, and endothelial cells (3, 5). Although IL-15 mRNA expression is observed in various tissues, detection of IL-15 protein in their culture supernatants has been extremely difficult. For example, IL-15 mRNA is induced in lipopolysaccharide/γ-interferon-activated monocytes, yet the supernatants as well as the lysates of such cells contain only minimal levels of IL-15 protein, suggesting that IL-15 production is regulated at multiple levels (5, 6). It has been proposed that this control is mainly posttranscriptional, i.e., at the level of protein translation and intracellular trafficking rather than transcriptional regulation, which is observed with many interleukins, including IL-2. Indeed, we have observed that IL-15 translation is heavily burdened by a number of elements; in particular by the presence of multiple AUGs and secondary structure in the 5′ untranslated region (5, 6). Furthermore, we previously addressed the negative regulation of IL-15 expression manifested by the unusually long signal peptide (LSP) (48 amino acid) and demonstrated that the coding sequence for this 48-aa signal peptide (SP) together with that for the mature protein of IL-15 impedes the translation of IL-15 protein in the COS expression system (unpublished data). In the current studies, we focused our efforts on the control of trafficking of IL-15 protein mediated by different SP isoforms. A splice variant of IL-15 was reported by two groups in a lung carcinoma cell line and T cell lines (7–9). Simultaneously we isolated a similar IL-15 isoform from a human testicular cDNA library. The mature cytokine of the new IL-15 isoform is identical to that of the published IL-15 cloned by Grabstein et al. (3). However, there is an insertion of an additional 119 nucleotides originating from intron 4 of the IL-15 genomic sequence, resulting in the removal of the original 48-aa SP and the generation of a new 21-aa SP. There are only 11 amino acids shared between these two signal sequences. Unexpectedly, IL-15 with the short signal peptide (SSP) was not secreted by transfected COS cells, prompting us to examine whether this finding is caused by reduced production of IL-15 (translational control) or by an alteration of intracellular IL-15 trafficking to a nonsecretory pathway (trafficking control). To address these questions, we defined systems where these two aspects of protein production control could be separated from each other. The use of an in vitro translation system enabled us to examine translational control exclusively. Trafficking/secretory control was examined by the generation of chimeric proteins consisting of the IL-15 SPs linked to green fluorescent protein (GFP) or IL-2. Here we report that this newly described SSP primarily controls the intracellular location of IL-15 by affecting intracellular trafficking, as compared with the observation that the 48-aa LSP predominantly affects the translation of IL-15 protein (unpublished data).
MATERIALS AND METHODS
Cloning of the Testicular IL-15 cDNA.
Human testicular cDNA library was purchased from Stratagene. One million clones were screened by using the human IL-15 cDNA corresponding to the full coding sequence. Positive clones were isolated after three cycles of plaque lifts. Nucleotide sequences of these clones were determined by Applied Biosystem’s 377 DNA sequencer (Perkin–Elmer).
RNA Blotting.
Total RNA was isolated from cynomolgus monkeys (maccaca fascicularis) by using the Purescript kit (Gentra Systems). Twenty micrograms of RNA was electrophoresed on a formaldehyde-agarose gel (1.2%) and transferred to the Hybond N+ membrane (Amersham). Blots were probed with a 32P-labeled human IL-15 fragment.
Protein Expression in COS Cells.
Ten micrograms of plasmid DNA was transfected into 4 million COS cells by electroporation (220 V, 960 μF).
IL-2/15 Biological Assay Using the CTLL-2 Cells.
The murine IL-2 dependent CTLL-2 cells were used as a biological indicator for IL-2 and IL-15 activity as previously described (10).
Immunoblotting.
Immunoblotting was performed as described previously (10). Polyclonal rabbit anti-human IL-15 antibody was kindly provided by Harmesh Sharma of Genzyme. Monoclonal rat anti-mouse IL-2 antibody was purchased from Genzyme. Polyclonal goat anti-mouse IL-2 antibody was purchased from Santa Cruz Biotechnology.
Constructs.
The GFP cDNA used to prepare fusion proteins was PCR-amplified from the pGreenLantern plasmid (Life Technologies, Gaithersburg, MD) with an elimination of the initiation ATG codon and addition of an MluI site (ACGCGT) in-frame. The murine IL-2 gene was PCR-amplified from cDNA synthesized by using mRNA from the mouse EL-4 cell line as a template, with the same modifications mentioned above. Oligonucleotides corresponding to the human IL-2 receptor α (IL-2Rα) SP, and human IL-15 SSP with the Kozak context and appropriate restriction ends were synthesized, annealed, and ligated to the GFP or IL-2 cDNA.
IL-2Rα SP was (only the sense oligonucleotide is shown): GGATCCACCATGGATTCATACCTGCTGATGTGGGGACTGCTCACGTTCATCATGGTGCCTGGCTGCCAGGCAACGCGT.
IL-15 SSP was (only the sense oligonucleotide is shown): GGATCCACCATGGTATTGGGAACCATAGATTTGTGCAGCTGTTTCAGTGCAGGGCTTCCTAAAACAGAAGCC.
The IL-15 LSP cDNA was amplified by PCR. PCR primers were: sense, GGATCCACCATGAGAATTTCGAAACCACATTTGAG; and antisense, ACGCGTGGCTTCTGTTTTAGGAAGCCC.
In Vitro Transcription/Translation.
For the in vitro translation assays, all of the fusion protein constructs were subcloned into the pSP64polyA vector (Promega) and linearized by EcoRI. In vitro transcription was performed by using Ambion’s Maxiscript kit with the SP6 RNA polymerase (Ambion, Austin, TX). Three hundred nanograms of the transcripts were introduced into either the wheat germ or the rabbit reticulocyte translation system according to the manufacturer’s instructions (Ambion) in the presence of [35S]methionine (Amersham). Translated proteins from the rabbit reticulocyte expression system were immunoprecipitated with a polyclonal antibody against IL-15 (Genzyme) or IL-2 (Santa Cruz) and electrophoresed. Gels were fixed and exposed to a PhosphorImaging screen (Molecular Dynamics).
RESULTS
Molecular Cloning of an Isoform of IL-15.
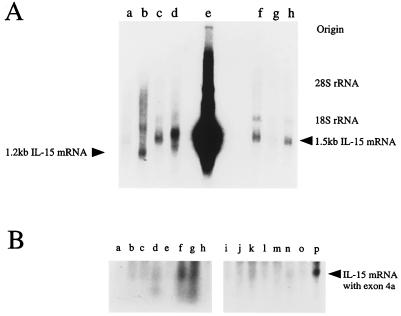
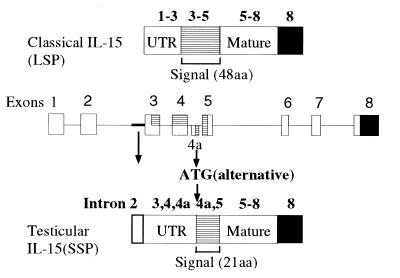
When we examined the tissue expression of IL-15, we observed that some tissues, especially the testis, expressed an aberrant-sized (1.2 kb) transcript (Fig. 1A) as compared with the 1.5-kb size seen with most tissues. To examine the nature of this transcript, we screened a human testicular cDNA library with IL-15 cDNA corresponding to the coding sequence. We obtained three clones designated 9A1, 9A2, and 11, that were shown to be identical after DNA sequencing. The 5′ untranslated region of these clones lacked the part of cDNA encoded by exons 1/2 (exon/intron numbering follows that of Anderson et al. in ref. 11). In the coding region for the SP, there was an additional 119-nt sequence inserted between exon 4 and 5. A search using the blast algorithm (National Center for Biological Information, Bethesda, MD) confirmed that this fragment is identical to the one present in the IL-15 sequence from a lung carcinoma cell line or T lymphoid cell lines published by other investigators (7–9). Comparison to the genomic sequence indicated that this 119-nt fragment originated from an element found in the intron 4 sequence. In this regard, this portion may be considered as a hidden additional exon (designated tentatively exon 4a). The introduction of the 119 nucleotides disrupts the 48-aa signal sequence of IL-15 by inserting a premature termination codon, and then provides an alternate initiation codon with a poor Kozak context (TTCATGG, ref. 12). Nonetheless when examined by an in vitro translation system, it was demonstrated that this ATG serves as an initiation codon. The alternate IL-15 form has a 21-aa N-terminal putative signal sequence as opposed to the 48-aa SP sequence of the classical IL-15 protein. Hydrophobicity analysis indicated that this alternative signal sequence does not have the typical characteristics of a signal peptide (7, 9). Based on the observation that this alternate IL-15 transcript contains a unique sequence derived from exon 4a, we next probed a tissue Northern blot (Invitrogen) with the exon 4a 119-nt fragment. As shown in Fig. 1B, the transcript containing the exon 4a was expressed in heart, thymus, and appendix. There was weak expression of this transcript seen in pancreas, testis, and gall bladder. This tissue distribution pattern differs from the one observed with the classical IL-15 mRNA, suggesting that the expressions of two IL-15 isoforms are differentially regulated. We tentatively designate the IL-15 isoform with the 21-aa SP as SSP-IL-15, and the one with the alternative 48-aa SP as LSP-IL-15. The diagram of the structures of these clones is indicated as Fig. 2.
Figure 1.
(A) Expression of a 1.2-kb IL-15 mRNA in testis. Total RNA from monkey tissues was electrophoresed, blotted onto Hybond N+ membrane, and probed by a DNA fragment of the human IL-15 entire coding region. Origin of RNA: a, kidney; b, testis; c, liver; d, pancreas; e, human HTLV-I(+) HuT 102 line; f, skeletal muscle; g, spleen; h, lung. HuT 102 expresses a chimeric IL-15 mRNA consisting of HTLV-I long terminal repeat and IL-15 (5) and this transcript migrates faster than the classical 1.5-kb IL-15 mRNA. Positions of 1.5-kb and 1.2-kb IL-15 transcripts are indicated. (B) Tissue expression of the mRNA encoding the SSP-IL-15. Human tissue blots (Northern Territory, Invitrogen) were probed by exon 4a fragment (see text for nomenclature). Origin of RNA: a, ovary; b, testis; c, prostate; d, gall bladder; e, lymphocytes; f, appendix; g, thymus; h, tonsil; i, skeletal muscle; j, spleen; k, pancreas; l, lung; m, liver; n, kidney; o, brain; p, heart.
Figure 2.
The structure of the testis-derived IL-15 clone 9A1. Genomic architecture of the human IL-15 gene (Middle), structures of the classical IL-15 (Top) and testicular IL-15 (Bottom) are shown. Numbers over each drawing indicates the exon numbering (11).
Production But Not Secretion of SSP-IL-15 Isoform by COS Cells.
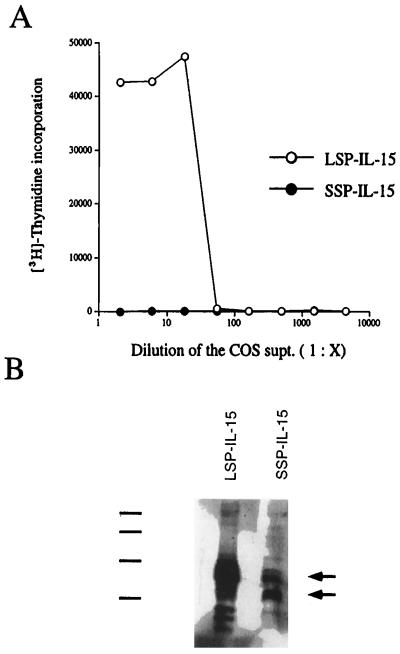
The SSP-IL-15 was subcloned into the pEF-Neo vector with human elongation factor-1α promoter, developed by Mayumi Naramura (Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, Rockville, MD) and was transfected into COS cells. Forty eight hours posttransfection, the COS supernatants were collected and subjected to the CTLL-2 proliferation assay. As shown in Fig. 3A, even at a 30-fold dilution of the COS supernatant, we could detect IL-15 activity in the supernatants of COS cells expressing the LSP-IL-15. In contrast, the SSP-IL-15-transfected COS cells did not manifest detectable activity in their supernatants, suggesting that either the protein is not translated or that it is not secreted. To address these alternatives, we performed immunoblotting of the COS lysates by using an anti-IL-15 polyclonal antibody. As shown in Fig. 3B, COS cells transfected with the SSP-IL-15 construct produced IL-15 protein intracellularly. However, compared with LSP-IL-15, the IL-15 band with the SSP-IL-15 construct showed less intensity, suggesting that either the SSP-IL-15 is less efficiently translated than the LSP-IL-15, that the SSP-IL-15 is more rapidly catabolized intracellularly, or that the SSP-IL-15 is sequestered in compartments that are not solubilized by Nonidet P-40 used to generate COS lysates. (see Fig. 5B).
Figure 3.
(A) IL-15 activity from COS supernatants transfected with LSP-IL-15 or SSP-IL-15 expression constructs. COS cells were transfected with the LSP-IL-15 (○), or the SSP-IL-15 (•) construct by electroporation, and the supernatants were collected 48 hr after transfection. The supernatants were tested by the CTLL-2 bioassay to measure IL-15 activity. (B) Immunoblotting of COS lysates expressing LSP-IL-15 or SSP-IL-15. COS cell lysates were blotted onto poly(vinylidene fluoride) membrane (Immobilon-P, Millipore) and probed with an anti-human IL-15 polyclonal antibody. The lower arrow indicates the fully processed SSP-IL-15, and the upper arrow indicates the IL-15 with the full 21-aa SSP. In the LSP-IL-15 lane, the multiple bands were observed because of glycosylation and the cleavage of the SP. Position of the protein size marker is indicated: 46 kDa, 33 kDa, 21.5 kDa, and 14.3 kDa, from top to bottom.
Figure 5.
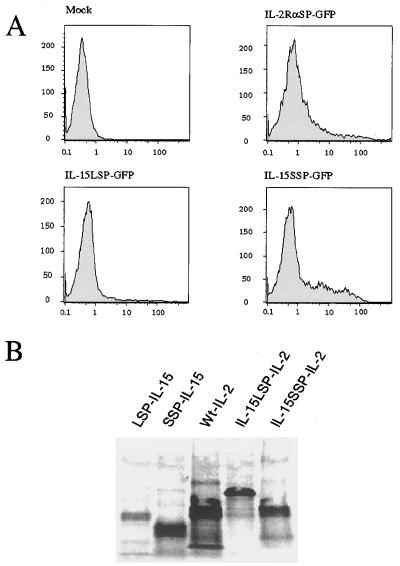
(A) Fluorescence of the COS cells transfected with various GFP constructs. COS cells were transfected with GFP constructs with various SPs (IL-2Rα SP, IL-15 LSP, and IL-15 SSP). After 48 hr, GFP fluorescence was analyzed by the FACSort (Becton–Dickinson) analyzer. (B) Rabbit reticulocyte in vitro translation using IL-15 or IL-2 constructs. Proteins were labeled with [35S]methionine by using rabbit reticulocyte in vitro translation system (Ambion), immunoprecipitated, and electrophoresed. Because the IL-15 LSP is longer (48 amino acids) than the IL-15 SSP (21 amino acids) or the wt IL-2 SP (21 amino acids), fusion proteins associated with the IL-15 LSP migrate slower than other proteins.
Generation of IL-2 Fusion Proteins Linked to the IL-15 Signal Peptides.
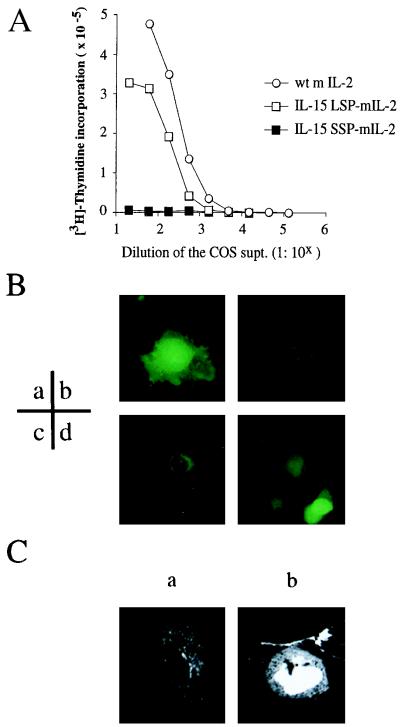
The COS transfection experiments suggested that the SSP of the IL-15 may affect the intracellular trafficking of proteins, resulting in the absence of IL-15 proteins in the supernatant. If this hypothesis were the case, the substitution of the wild-type (wt) SP of other proteins by the SSP should interfere with secretion of these proteins. To test this hypothesis, we generated a set of fusion constructs consisting of the mouse IL-2 mature protein cDNA linked to cDNAs coding for various signal peptides. These constructs then were transfected into COS cells, and the supernatants were collected and tested by the CTLL-2 assay. In Fig. 4A we show that the cells transfected with the wt IL-2 construct produced 3- to 5-fold more IL-2 protein (a 300-fold diluted specimen achieving 50% maximum proliferative response) than the IL-15 LSP-IL-2 transfectants. The mouse IL-2 standard used in this assay achieved an ED50 value at 15 pM concentration, suggesting that there was roughly 4.5 nM of IL-2 produced by the COS cells with the wt IL-2 construct. In contrast, the supernatants of cells expressing the IL-15 SSP-IL-2 showed no CTLL-2 activity even at the highest concentration [50% (vol/vol) COS supernatant in the CTLL-2 culture], suggesting that the substitution of the SSP abolished secretion of the IL-2 protein. We also performed immunoblotting of the transfected COS lysates by using an anti-mouse IL-2 polyclonal (Santa Cruz Biotechnology) and identified the intracellular production of IL-2 by the COS cells transfected with the IL-15 SSP-IL-2 construct (data not shown). Thus the absence of IL-2 activity in the COS supernatant of IL-15 SSP-IL-2 transfectant was not because of the lack of cytokine production, but rather was because of the effects of the substitution of wt IL-2 SP with IL-15 SSP on intracellular trafficking and secretion.
Figure 4.
(A) IL-2 activity detected in the supernatant of COS cells transfected with IL-15 LSP-IL-2, IL-15 SSP-IL-2, or wt IL-2. COS cells transfected the murine IL-2 (mIL-2) constructs with wt IL-2 SP (○), IL-15 LSP (□), or the IL-15 SSP (▪) were cultured for 48 hr. The supernatants of the transfected COS cells were serially diluted and assessed by using the murine CTLL-2 cell line for their secreted IL-2 activity. (B) Immunofluorescence of the COS cells expressing GFP proteins linked with various signal peptides. COS cells transfected with the GFP constructs with various SPs (a, no SP; b, IL-2Rα SP; c, IL-15 LSP; d, IL-15 SSP) were analyzed for their fluorescence by inverted immunofluorescence microscopy. (C) Confocal microscopic analysis of the COS cells expressing the IL-15 LSP-GFP or the IL-15 SSP-GFP protein. COS cells transfected the IL-15 LSP-GFP or the IL-15 SSP-GFP constructs were fixed and analyzed by scanning confocal immunofluorescence microscopy. (a) COS cells transfected with the IL-15 LSP-GFP construct; (b) COS cells transfected with the IL-15 SSP-GFP construct.
Generation of Fusion Proteins Composed of the IL-15 SPs and the GFP.
So far, we have demonstrated that IL-15 with the SSP is produced intracellularly, but is not secreted. To examine to which compartment the proteins are transported, we fused either the LSP (48-aa SP of IL-15) or SSP (21-aa SP of IL-15) with the Aequorea victoria GFP. The LSP-GFP, SSP-GFP, and wt GFP constructs were transfected into COS cells together with a GFP construct that was fused with the IL-2Rα SP (IL-2Rα SP-GFP) as the control for a protein directed to the secretory pathway. The fluorescence of the cells manifested by the GFP was analyzed by immunofluorescence microscopy 48 hr after transfection. As shown in Fig. 4B-b, a typical secretory pattern was observed with IL-2Rα SP-GFP, in that there was endoplasmic reticulum (ER) staining. With IL-15 LSP-GFP, the pattern was more complicated. There was cytoplasmic as well as nuclear staining observed with about 15% of the fluorescent cells, although the majority of the cells (85% of the cells with fluorescence) showed the ER staining pattern (Fig. 4B-c). In contrast, the SSP-GFP containing cells displayed cytoplasmic as well as nuclear staining (Fig. 4B-d). To further investigate the distribution of GFP in detail, we used confocal microscopic examination. As shown in Fig. 4C-a, the LSP-GFP displayed ER staining as the major pattern with nuclear staining seen in some cells (data not shown), whereas the SSP-GFP was seen in the cytoplasm as well as the nucleus (Fig. 4C-b). The SSP-GFP fluorescence pattern is in good accord with the observation that no IL-15 biological activity was detectable in the supernatant of COS cells transfected with the SSP-IL-15 construct. Taken together, these results suggest that the SSP-IL-15 is designed to be stored intracellularly.
Assessment of the Effect of IL-15 SSP on the Translational Impediment of the IL-15 Protein.
We have demonstrated that the LSP has a negative effect on IL-15 translation (unpublished data). To examine if this effect is also the case with the SSP, we analyzed COS cells transfected with LSP, SSP, and IL-2Rα SP-GFP constructs by flow cytometry. As shown in Fig. 5A, we observed a marked decrease of fluorescence only with the cells transfected with the LSP-GFP, but not with the SSP-GFP construct as compared with that with the cells transfected with the IL-2Rα SP-GFP construct. (Fig. 5A). Earlier, we raised three alternative possibilities to account for the immunoblotting result shown in Fig. 3B, and it seems likely that the SSP-IL-15 was produced in larger amount than the LSP-IL-15, and that Nonidet P-40 used to lyse the COS cells did not effectively solubilize the SSP-IL-15 protein, possibly because it was stored in the nucleus. We also carried out in vitro translation experiments by using two different systems. In the wheat germ system, we did not observe noticeable differences among all the constructs tested (data not shown) in terms of the amount of the proteins generated, suggesting that the cRNA derived from each construct is capable of producing equal amount of proteins in a system where the translational impediment does not occur (unpublished data). However, with the rabbit reticulocyte translation system, we observed that the amount of LSP-IL-15 expressed was much smaller than that with the other translated proteins including the SSP-IL-15 (Fig. 5B), indicating that the translational impediment as assessed in this in vitro system is a characteristic that is solely attributable to the LSP, but not to the SSP. It is also notable that IL-2 with the IL-15 LSP showed a similar decrease in translation, although the magnitude of this decrease seems less than what was observed with IL-15 proteins. Previously, we reported that the replacement of wt IL-2 SP with IL-15 LSP reduced the production of human IL-2 in COS cells by 50-fold (unpublished data). This reduction is much larger than the one observed here with mouse constructs. However, we noticed that human IL-2 is more readily translated than mouse IL-2 in COS system (2- to 3-fold). It should be noted as well that in the previous occasion, we used IL-2 and IL-15 constructs with their native initiation sequences intact (non-Kozak consensus) whereas all the constructs used in this study have the Kozak consensus context (ACCATG, ref. 12). With IL-15, this change enhances the protein production by 3- to 5-fold. With IL-2, to our surprise, this change reduced the IL-2 production by several fold (R.N.B., unpublished observation). The combinatorial effects of all of these elements seem to account for the difference we observed with the two systems, suggesting that the translational control of IL-15 is pretty complex but is attributable to the IL-15 LSP. In summary, the LSP of IL-15 directs the production of IL-15 that is largely secreted although the overall production is impeded at the translational level in a very complicated manner. On the other hand, the SSP directs the production of an intracellular form of IL-15 without a significant impediment to translation of the IL-15 protein.
DISCUSSION
In this study, we have demonstrated that there are at least two isoforms of pre-IL-15 proteins. The one with a 48-aa SP (LSP) is expressed largely in tissues such as skeletal muscle, placenta, heart, lung, liver, thymus, and kidney (3). This isoform is observed in the ER and appears to be largely secreted. The alternative form with a SSP is not secreted by COS cells, but localizes primarily diffusely in the cytoplasm as well as in the nucleus. This form is expressed in heart, thymus, appendix, and testis. This SSP-IL-15 isoform is identical to that observed in a tumor cell line and T cell lines recently reported by two other groups (7–9). These groups as well as our own observed the lack of secretion of the SSP-IL-15 by using a COS transient transfection system.
Microscopic observations using the GFP constructs indicated that the SSP-IL-15 isoform is not directed to the secretory pathway because we did not observe the presence of SSP-GFP in the ER. The distribution of this form in the nucleus is interesting and even suggests the presence of a possible partner there. Interestingly, IL-15Rα, the specific IL-15 binding subunit of the T/NK type I IL-15 receptor, possesses a potential nuclear localization signal in its SP (J.A.H., unpublished observation). In addition, IL-15Rα by itself has an extremely high affinity (Kd: ca 100 pM) for IL-15 (13), almost 100-fold higher than the affinity of the IL-2Rα for IL-2. Collectively, these data may suggest that the nuclear IL-15 is retained there because of the presence of the IL-15Rα in the nucleus. However, our preliminary results indicate that this mechanism for nuclear localization of IL-15 is unlikely to be the case, in that we did not observe nuclear localization of IL-15Rα when a construct encoding this protein was transfected into COS cells (G.K., unpublished observation).
In interpreting the nuclear localization of the SSP-IL-15, we need to consider the possibility that such nuclear staining could be the result of passive diffusion of the small IL-15 protein (15 kDa) from the cytoplasm. However, that the nuclear staining seems much brighter than that of the adjacent cytoplasmic portion of COS cells expressing the SSP-GFP suggests that the nuclear staining we observed is a result of an active process that involves special trafficking of the IL-15 protein with the SSP.
The SSP-IL-15 and LSP-IL-15 show distinct tissue distribution, in that only LSP-IL-15 mRNA, but not SSP-IL-15 mRNA, is expressed in some tissues such as skeletal muscle and kidney. Nevertheless, with several cell lines a parallel expression of both species was observed (7, 9). The lack of exons 1/2 and the presence of a 219-nt fragment that originated from intron 2 in the 5′ untranslated region of the SSP-IL-15 transcript indicates that this isoform may be transcriptionally regulated by an alternate enhancer/promoter in intron 2. Although these hypotheses require further investigation, the size of the SSP-IL-15 cDNA (1.2 kb) is in good accord with that of the transcript observed in testis (Fig. 1A), indicating that the cDNA we isolated is close to full length. Furthermore, we observed that the unusually long intron 2 (10 kb, ref. 11) contains a potential initiation site as well as enhancer-like motifs (N.A., unpublished observation). Thus, it is likely that the two isoforms of IL-15 are generated by the usage of alternate promoters, rather than being generated by alternative splicing, which seems consistent with the notion that the two IL-15 isoforms serve different roles.
Sorting of the same protein to different cellular compartments by modifying the regulatory sequence has been observed in other systems as well. Examples include proteins such as stem cell factor (SCF, ref. 14) and Int-2, a fibroblast growth factor-related oncoprotein (15). The case with the SCF involves the elimination of the membrane anchorage portion from the protein, thus rendering the SCF either a soluble or membrane-bound molecule. In the case for Int-2, two different signal peptides are generated by the usage of different start codons in-frame, resulting in the transporting of the protein to either the secretory pathway or the nucleus. The IL-15 case seems very similar to that of Int-2.
It has been demonstrated that the LSP-IL-15 is produced inefficiently as a secretory protein by mammalian cells, by cultured cell lines and the COS transient expression system (unpublished data). Meazza et al. (8) and Onu et al. (9) reported that no IL-15 protein could be detected with the LSP-IL-15 when supernatants from transfected COS-7 cells were assayed by the CTLL-2 biological assay. Our data are consistent with theirs in that IL-15 translation and secretion is inefficient, almost 2–3 logs lower than that of a more typical lymphokine such as IL-2 or IL-4. However, in our studies, we could demonstrate the production of LSP-IL-15 as a secreted protein when we used the COS-7 transfection system (Fig. 3A; ref. 5). The discrepancy between the studies may be because of differences in the sensitivity of the CTLL-2 cell clones and the transfection efficiency of the COS-7 systems used. In addition, we observed that several nontransfected cell lines produce and secrete IL-15 protein into their supernatants, achieving levels that were detectable by both bioassay and IL-15 ELISA. The amount of the IL-15 mRNA in these cells is generally much lower than that observed in transfected COS cells so that it could be detected by Northern analysis only with poly(A)-enriched RNA preparations (Y.T., unpublished observation). It is plausible that appropriate stimuli such as intracellular infection of appropriate cells enhances IL-15 translation by circumventing the translational impediments caused by the 48-aa SP and the 5′ untranslated region. The mechanism underlying the unburdening of IL-15 translation is an important issue in defining the physiological functions of IL-15. In some cases, we could detect IL-15 protein by ELISA in the sera from healthy donors, suggesting that IL-15 protein is actually produced in vivo (G.K., unpublished observation).
Recent observations suggested that IL-15 is critical in NK cell development in the bone marrow (16–19). Furthermore, we recently obtained evidence that functional elimination of the IL-15 expression can lead to the loss of NK cells (20). Taken together, it is reasonable to assume that there are circumstances where secretable LSP-IL-15 is produced in vivo either physiologically or pathologically. Bone marrow stromal cells, monocytes, or dendritic cells infected with intracellular organisms such as Mycobacterium leprae (21) would be a good place to search for such a mechanism to unleash the translational impediments posed on LSP-IL-15 production.
It is interesting that there is little translational control mediated by the SSP (Fig. 5 A and B). It is well known that the production of IL-2 is strictly controlled, to ensure that the immune cell will bind this factor only after a challenge by foreign antigens. If the production of IL-15 were not prevented by translational controls, the widespread expression of IL-15 transcripts would lead to the constitutive production of this protein, thereby disrupting the homeostasis of the immune system. Indeed, it has been suggested that such uncontrolled expression of IL-15 might be associated with inflammatory or autoimmune disease (22), because secreted IL-15 stimulates tumor necrosis factor α (23) and IL-1β production as well as T and NK cell activation. As the SSP-IL-15 does not exist outside of the producer cells, it is logical that the expression of this isoform does not need to be tightly regulated. Such intracellular IL-15 may play a novel as yet undefined role within the cells that produced it. Alternatively the intracellular form may serve as a reservoir of IL-15 protein being released on damage and destruction of the producer cells when they are challenged by cytotoxic cells.
Acknowledgments
We thank Dr. Mayumi Naramura of the Laboratory of Immunology, National Institute of Allergy and Infectious Diseases for the kind supply of the pEF-Neo expression vector, and Dr. Harmesh Sharma of Genzyme for providing the anti-human IL-15 polyclonal antibody.
ABBREVIATIONS
- IL
interleukin
- GFP
green fluorescent protein
- IL-2R
IL-2 receptor
- wt
wild type
- SP
signal peptide
- ER
endoplasmic reticulum
- LSP
long signal peptide
- SSP
short signal peptide
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF031167).
References
- 1.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn A, Ahdieh M, Johnson L, Alderson M R, Watson J D, Anderson D M, Giri J G. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 4.Giri J G, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park L S, Cosman D, Anderson D. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamford R N, Battiata A P, Burton J D, Sharma H, Waldmann T A. Proc Natl Acad Sci USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tagaya Y, Bamford R N, DeFilippis A P, Waldmann T A. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 7.Meazza R, Verdiani S, Biassoni R, Coppolecchia M, Gaggero A, Orengo A M, Colombo M P, Azzarone B, Ferrini S. Oncogene. 1996;16:2187–2192. [PubMed] [Google Scholar]
- 8.Meazza R, Gaggero A, Neglia F, Basso S, Sforzini S, Pereno R, Azzarone B, Ferrini S. Eur J Immunol. 1997;27:1049–1054. doi: 10.1002/eji.1830270502. [DOI] [PubMed] [Google Scholar]
- 9.Onu A, Pohl T, Krause H, Bulfone-Paus S. J Immunol. 1997;158:255–262. [PubMed] [Google Scholar]
- 10.Tagaya Y, Miyamoto Y, Burton J D, Waldmann T A. EMBO J. 1996;18:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson D M, Johnson L, Glaccum M B, Copeland N G, Gilbert D J, Jenkins N A, Valentine V, Kirstein M N, Shapiro D N, Morris S W, Grabstein K, Cosman D. Genomics. 1995;25:701–706. doi: 10.1016/0888-7543(95)80013-c. [DOI] [PubMed] [Google Scholar]
- 12.Kozak M. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanagan J G, Chan D C, Leder P. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 15.Acland P, Dixon M, Peters G, Dickson C. Nature (London) 1990;343:662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- 16.Carson W E, Fehniger T A, Haldar S, Eckhert K, Lindemann M J, Lai C-F, Croce C M, Baumann H, Caligiuri M A. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrózek E, Anderson P, Caligiuri M A. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 18.Puzanov I J, Bennett M, Kumar V. J Immunol. 1996;157:4282–4285. [PubMed] [Google Scholar]
- 19.Puzanov I J, Williams N S, Schatzle J, Sivakumar P V, Bennett M, Kumar V. Res Immunol. 1997;148:195–201. doi: 10.1016/s0923-2494(97)84225-3. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara, K., Hida, S., Azimi, N., Tagaya, Y., Sato, T., Yokochi-Fukuda, T., Waldmann, T. A., Taniguchi, T. & Taki, S., Nature (London), in press. [DOI] [PubMed]
- 21.Jullien D, Sieling P A, Uyemura K, Mar N D, Rea T H, Modlin R L. J Immunol. 1997;158:800–806. [PubMed] [Google Scholar]
- 22.Kirman I, Nielsen O H. Am J Gasteroenterol. 1996;91:1789–1794. [PubMed] [Google Scholar]
- 23.McInnes I B, Leung B P, Sturrock R D, Field M, Liew F Y. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]