Abstract
The factors responsible for the regulation of protein synthesis in the zoospores of Blastocladiella emersonii were studied by means of cell fractionation and in vitro assays. Charged transfer ribonucleic acid (tRNA) and aminoacyl-tRNA synthetases were found both inside the membrane-bound, ribosomal nuclear cap, and in the extracap cytoplasm. Ribosomes isolated from zoospore nuclear caps in low salt buffer failed to support polyuridylic acid-dependent phenylalanine incorporation. After washing with high salt buffer, the cap ribosomes were equivalent in activity to similarly prepared plant ribosomes. Both the high-salt wash from cap ribosomes and the extracap supernatant fraction contained an unidentified material which inhibited aminoacyl-tRNA binding and peptide bond formation by ribosomes. Ribosomal binding of polyuridylic acid was not inhibited. Washed cap ribosomes supported very low incorporation rates without added messenger RNA, and were highly dependent upon added poly U for phenylalanine incorporation, indicating a low level of messenger in nuclear caps. It is concluded that enclosure of the ribosomes in the nuclear cap does not in itself prevent protein synthesis, and that the lack of activity may be due to the presence of a ribosome inhibitor.
Full text
PDF


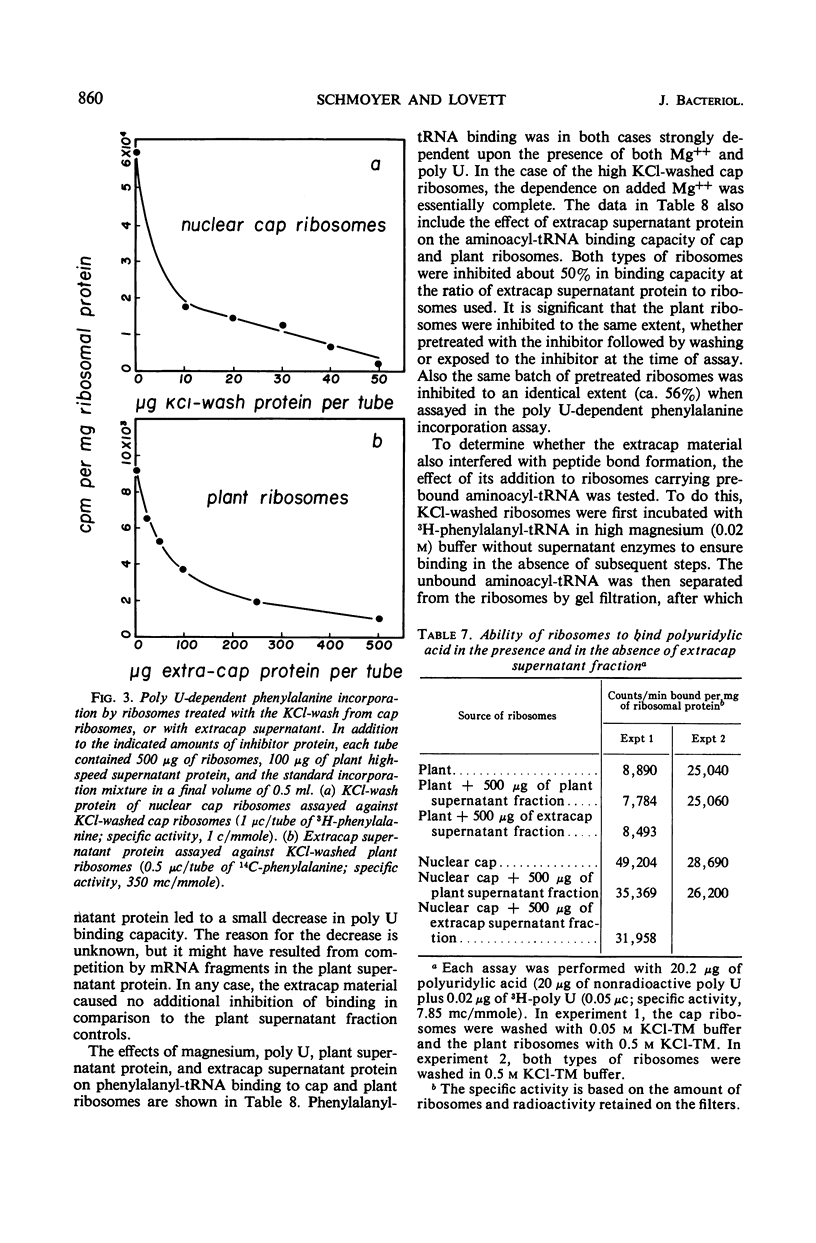


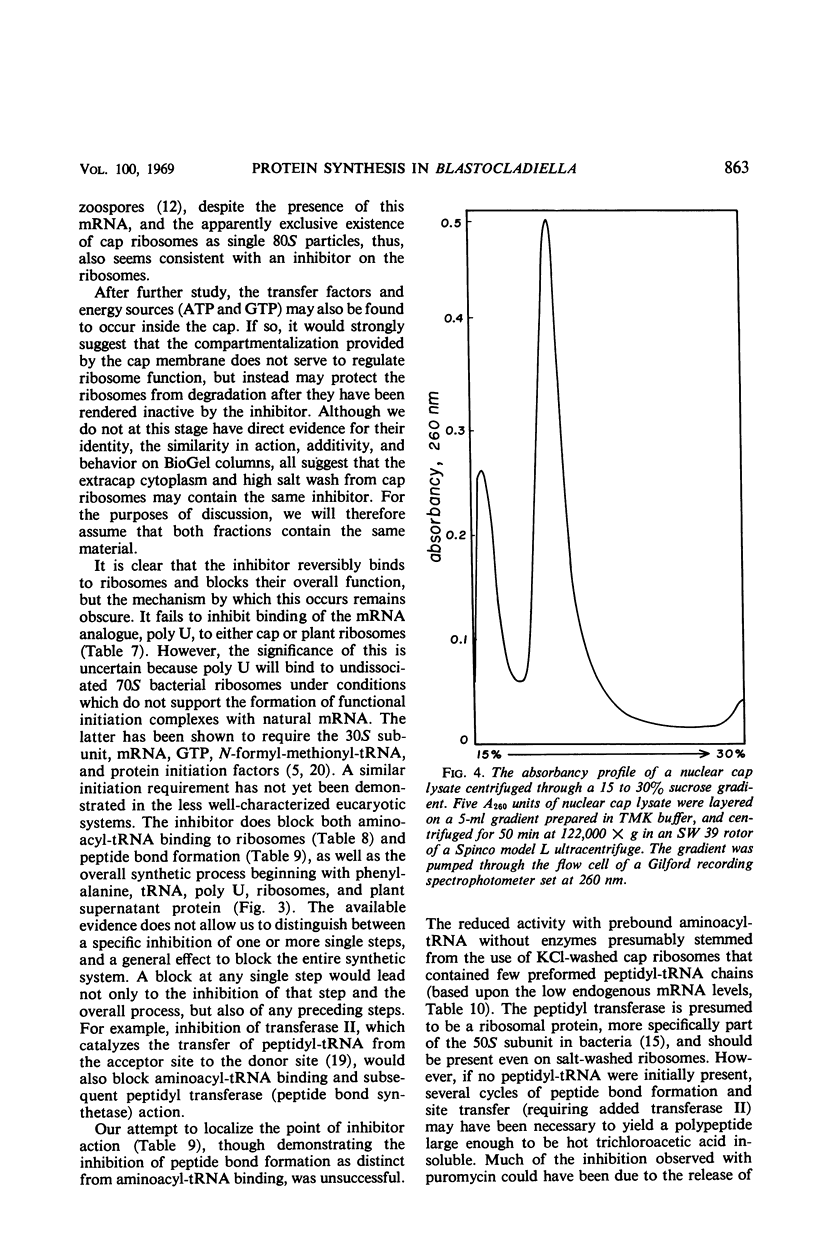

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A., Faiman L. E., Neidhardt F. C. Biochemical and genetic characterization of a mutant of Escherichia coli with a temperature-sensitive valyl ribonucleic acid synthetase. J Bacteriol. 1966 Oct;92(4):1076–1082. doi: 10.1128/jb.92.4.1076-1082.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., KATZ S. STUDIES ON THE BIOSYNTHESIS AND METHYLATION OF TRANSFER RNA. J Mol Biol. 1964 Jun;8:790–800. doi: 10.1016/s0022-2836(64)80160-1. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Chambon P., Konberg A. Biochemical studies of bacterial sporulation and germination. XI. Protein-synthesizing systems from vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5117–5125. [PubMed] [Google Scholar]
- Guthrie C., Nomura M. Initiation of protein synthesis: a critical test of the 30S subunit model. Nature. 1968 Jul 20;219(5151):232–235. doi: 10.1038/219232a0. [DOI] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Studies on the conidia of Aspergillus oryzae. VII. Development of protein synthesizing activity during germination. Biochim Biophys Acta. 1968 Sep 24;166(2):505–511. [PubMed] [Google Scholar]
- Kobayashi Y., Halvorson H. O. Evidence for a defective protein synthesizing system in dormant spores of Bacillus cereus. Arch Biochem Biophys. 1968 Mar 11;123(3):622–632. doi: 10.1016/0003-9861(68)90182-3. [DOI] [PubMed] [Google Scholar]
- LOVETT J. S. CHEMICAL AND PHYSICAL CHARACTERIZATION OF "NUCLEAR CAPS" ISOLATED FROM BLASTOCLADIELLA ZOOSPORES. J Bacteriol. 1963 Jun;85:1235–1246. doi: 10.1128/jb.85.6.1235-1246.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie P. E., Lovett J. S. Ultrastructural changes during sporangium formation and zoospore differentiation in Blastocladiella Emersonii. Am J Bot. 1968 Feb;55(2):220–236. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. S. Reactivation of ribonucleic acid and protein synthesis during germination of Blastocladiella zoospores and the role of the ribosomal nuclear cap. J Bacteriol. 1968 Oct;96(4):962–969. doi: 10.1128/jb.96.4.962-969.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Monro R. E. Catalysis of peptide bond formation by 50 S ribosomal subunits from Escherichia coli. J Mol Biol. 1967 May 28;26(1):147–151. doi: 10.1016/0022-2836(67)90271-9. [DOI] [PubMed] [Google Scholar]
- Moore P. B. Polynucleotide attachment to ribosomes. J Mol Biol. 1966 Jun;18(1):8–20. doi: 10.1016/s0022-2836(66)80072-4. [DOI] [PubMed] [Google Scholar]
- Murphy M. N., Lovett J. S. RNA and protein synthesis during zoospore differentiation in synchronized cultures of Blastocladiella. Dev Biol. 1966 Aug;14(1):68–95. doi: 10.1016/0012-1606(66)90006-6. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. The interrelationship between guanosine triphosphatase and amino acid polymerization. Arch Biochem Biophys. 1966 Sep 26;116(1):344–351. doi: 10.1016/0003-9861(66)90040-3. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Translation of the genetic message. Naturwissenschaften. 1968 Nov;55(11):505–514. doi: 10.1007/BF00660121. [DOI] [PubMed] [Google Scholar]
- Söll D., Jones D. S., Ohtsuka E., Faulkner R. D., Lohrmann R., Hayatsu H., Khorana H. G. Specificity of sRNA for recognition of codons as studied by the ribosomal binding technique. J Mol Biol. 1966 Aug;19(2):556–573. doi: 10.1016/s0022-2836(66)80023-2. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Brambl R. M. Protein synthesis during fungal spore germination. II. Aminoacyl-soluble ribonucleic acid synthetase activities during germination of Botryodiplodia theobromae spores. J Bacteriol. 1968 Oct;96(4):1042–1048. doi: 10.1128/jb.96.4.1042-1048.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L. Protein synthesis during fungal spore germination. I. Characteristics of an in vitro phenylalanine incorporating system prepared from germinated spores of Botryodiplodia theobromae. Arch Biochem Biophys. 1968 Apr;125(1):13–21. doi: 10.1016/0003-9861(68)90632-2. [DOI] [PubMed] [Google Scholar]



