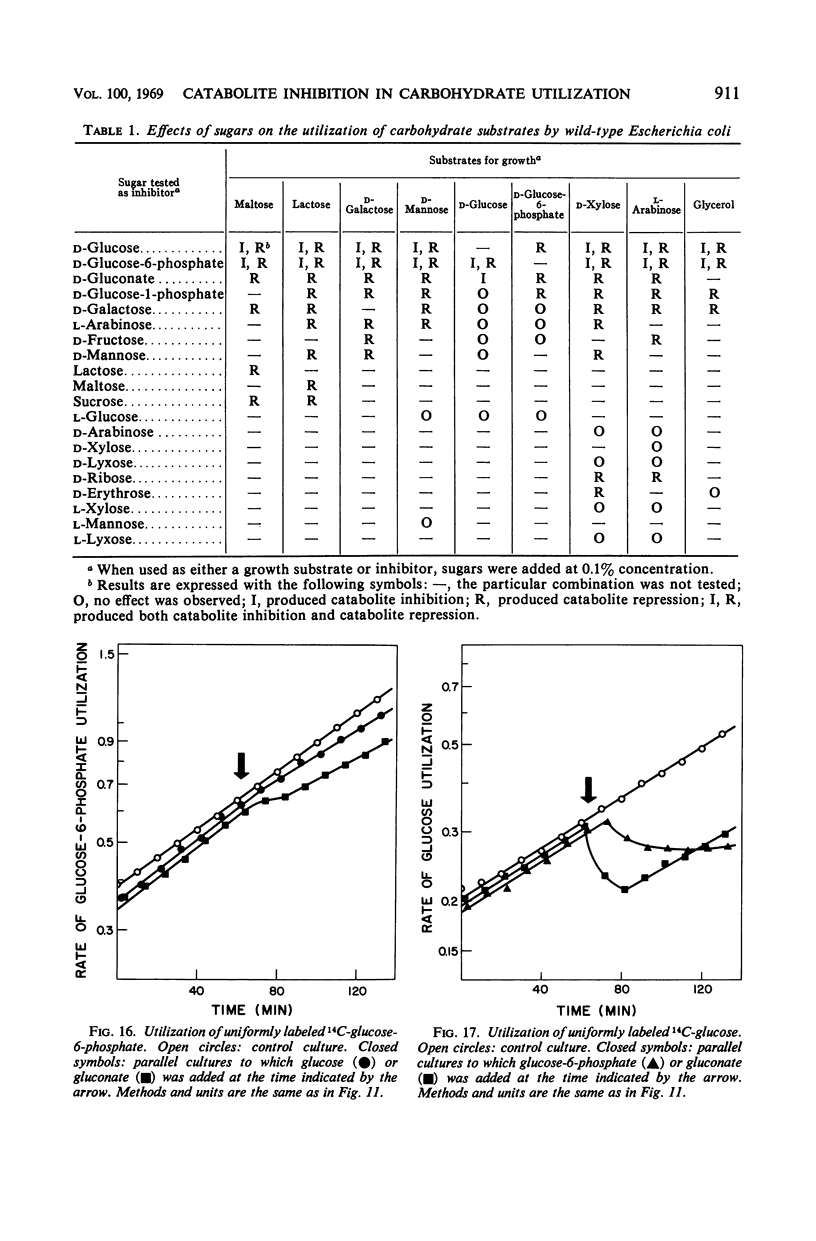
Abstract
When Escherichia coli is grown in synthetic medium with radioactive galactose or lactose as the carbon source, the addition of glucose rapidly inhibited utilization of the radioactive substrate, whether the formation of 14CO2 or acid-insoluble products was measured. The inhibition was reversed after the removal of glucose. Experiments with mutants blocked in subsequent steps of galactose and lactose metabolism demonstrated that the inhibition occurs prior to the formation of the first metabolic product. The utilization of a variety of sugars, including maltose, lactose, mannose, galactose, l-arabinose, xylose, and glycerol was inhibited by glucose. Of a number of carbohydrates tested as potential inhibitors, only glucose and, to a lesser extent, glucose-6-phosphate (G-6-P) were capable of inhibiting the utilization of all of the substrates. Glucose did not inhibit G-6-P utilization but G-6-P inhibited glucose utilization. With all substrates, except glycerol, there was a delay before the onset of inhibition by G-6-P. We conclude that E. coli has a general regulatory mechanism, termed catabolite inhibition, which controls the activity of early reactions in carbohydrate metabolism, allowing certain substrates to be utilized preferentially.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Guest J. R. Deferred metabolism of glucose by Clostridium tetanomorphum. J Gen Microbiol. 1968 Dec;54(2):277–286. doi: 10.1099/00221287-54-2-277. [DOI] [PubMed] [Google Scholar]
- Blackkolb F., Schlegel H. G. Katabolische Repression und Enzymhemmung durch molekularen Wasserstoff bei Hydrogenomonas. Arch Mikrobiol. 1968;62(2):129–143. [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- GAUDY A. F., Jr, GAUDY E. T., KOMOLRIT K. Multicomponent substrate utilization by natural populations and a pure culture of Escherichia coli. Appl Microbiol. 1963 Mar;11:157–162. doi: 10.1128/am.11.2.157-162.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. I. General properties as studied in a galactokinaseless mutant. J Biol Chem. 1960 Jun;235:1580–1585. [PubMed] [Google Scholar]
- KESSLER D. P., RICKENBERG H. V. The competitive inhibition of alpha-methylglucoside uptake in Escherichia coli. Biochem Biophys Res Commun. 1963 Mar 25;10:482–487. doi: 10.1016/0006-291x(63)90383-8. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogell B. M., Maity B. R., Frumkin S., Shapiro S. Induction of an active transport system for glucose 6-phosphate in Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):406–415. doi: 10.1016/0003-9861(66)90047-6. [DOI] [PubMed] [Google Scholar]
- Stumm-Zollinger E. Effects of inhibition and repression on the utilization of substrates by heterogeneous bacterial communities. Appl Microbiol. 1966 Jul;14(4):654–664. doi: 10.1128/am.14.4.654-664.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Convery H. J., Lea M. A., Stamm N. B. Feedback inhibition of key glycolytic enzymes in liver: action of free fatty acids. Science. 1966 Dec 9;154(3754):1357–1360. doi: 10.1126/science.154.3754.1357. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]


