Abstract
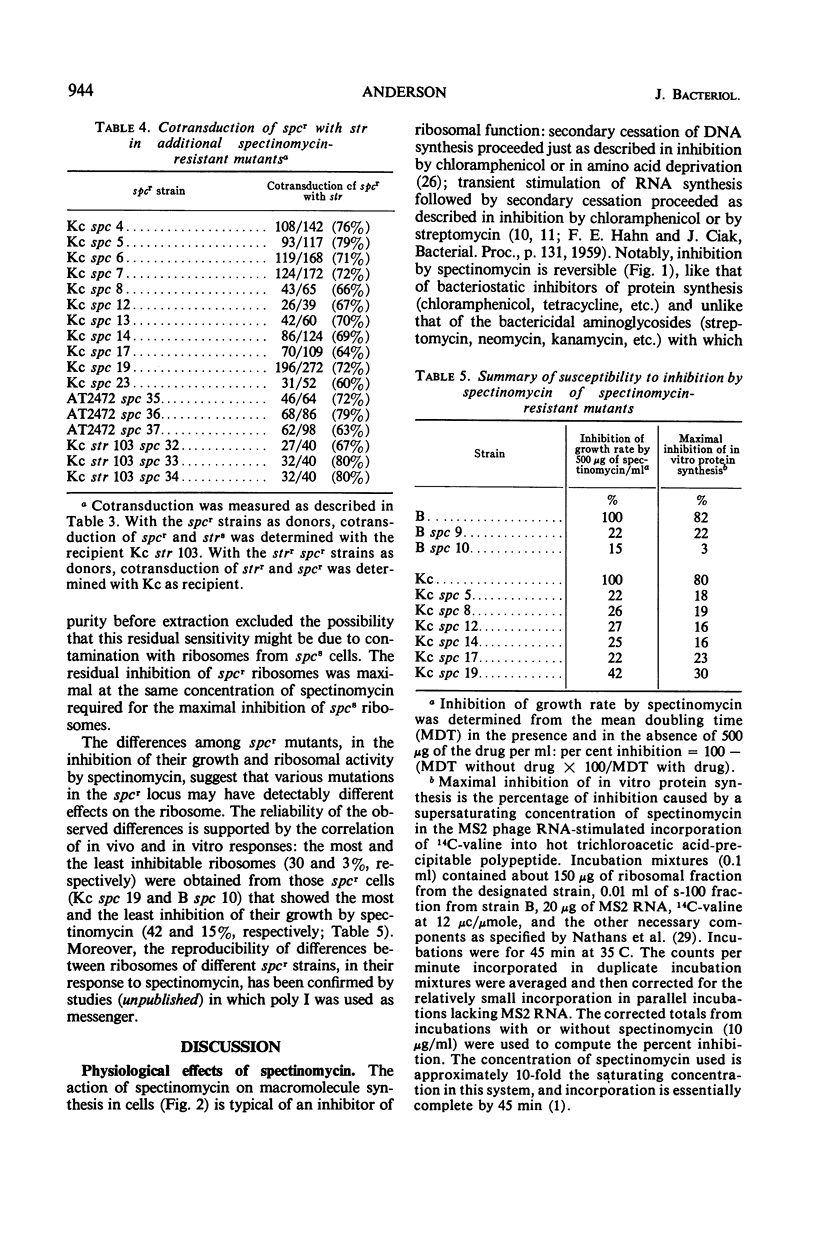
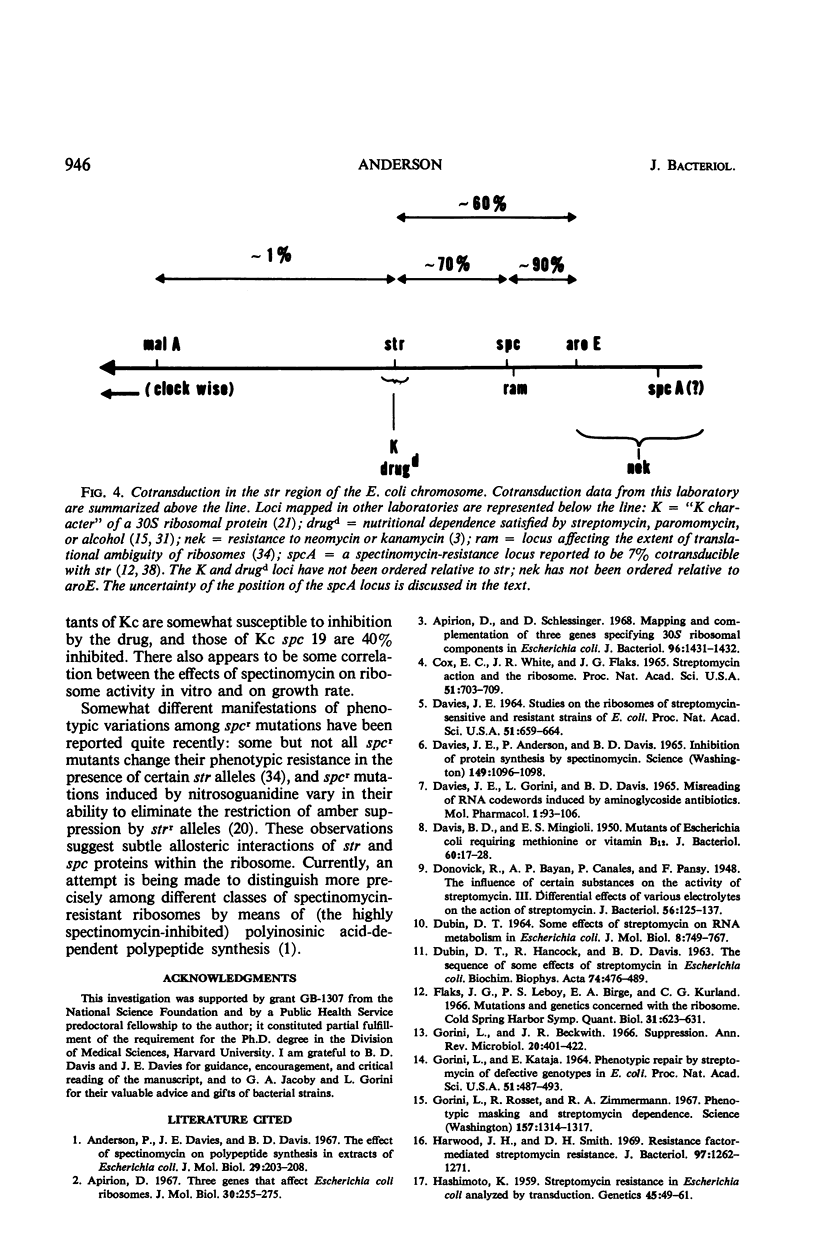
Inhibition of growth and division of Escherichia coli by spectinomycin is reversible, and the kinetics of its interference with deoxyribonucleic and ribonucleic acid synthesis may be interpreted as secondary effects of inhibition of protein synthesis on the ribosome. Spontaneous mutations to spectinomycin resistance occur in E. coli K-12 at a rate of about 2 × 10−10. Resistance is transducible with a discrete lag in phenotypic expression, and the kinetics of its development is about the same as that for streptomycin resistance. All spectinomycin-resistant mutants tested contain resistant ribosomes, and all map in a locus (spc) counterclockwise to and 70% cotransducible with the classical str locus. Differences in the residual drug sensitivity of various spectinomycin-resistant mutants, and of their ribosomes, indicate the existence of more than one phenotypic class of resistance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Davies J., Davis B. D. Effect of spectinomycin on polypeptide synthesis in extracts of Escherichia coli. J Mol Biol. 1967 Oct 14;29(1):203–215. doi: 10.1016/0022-2836(67)90191-x. [DOI] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Mapping and complementation of three genes specifying 30S ribosomal components in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1431–1432. doi: 10.1128/jb.96.4.1431-1432.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- COX E. C., WHITE J. R., FLAKS J. G. STREPTOMYCIN ACTION AND THE RIBOSOME. Proc Natl Acad Sci U S A. 1964 Apr;51:703–709. doi: 10.1073/pnas.51.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., HANCOCK R., DAVIS B. D. THE SEQUENCE OF SOME EFFECTS OF STREPTOMYCIN IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 13;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- DUBIN D. T. SOME EFFECT OF STREPTOMYCIN ON RNA METABOLISM IN ESCHERICHIA COLI. J Mol Biol. 1964 May;8:749–767. doi: 10.1016/s0022-2836(64)80122-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Anderson P., Davis B. D. Inhibition of protein synthesis by spectinomycin. Science. 1965 Sep 3;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- Davies J., Gorini L., Davis B. D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol. 1965 Jul;1(1):93–106. [PubMed] [Google Scholar]
- Donovick R., Bayan A. P., Canales P., Pansy F. The Influence of Certain Substances on the Activity of Streptomycin: III. Differential Effects of Various Electrolytes on the Action of Streptomycin. J Bacteriol. 1948 Jul;56(1):125–137. [PMC free article] [PubMed] [Google Scholar]
- Flaks J. G., Leboy P. S., Birge E. A., Kurland C. G. Mutations and genetics concerned with the ribosome. Cold Spring Harb Symp Quant Biol. 1966;31:623–631. doi: 10.1101/sqb.1966.031.01.081. [DOI] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. PHENOTYPIC REPAIR BY STREPTOMYCIN OF DEFECTIVE GENOTYPES IN E. COLI. Proc Natl Acad Sci U S A. 1964 Mar;51:487–493. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L., Beckwith J. R. Suppression. Annu Rev Microbiol. 1966;20:401–422. doi: 10.1146/annurev.mi.20.100166.002153. [DOI] [PubMed] [Google Scholar]
- Gorini L., Rosset R., Zimmermann R. A. Phenotype masking and streptomycin dependence. Science. 1967 Sep 15;157(3794):1314–1317. doi: 10.1126/science.157.3794.1314. [DOI] [PubMed] [Google Scholar]
- Harwood J. H., Smith D. H. Resistance factor-mediated streptomycin resistance. J Bacteriol. 1969 Mar;97(3):1262–1271. doi: 10.1128/jb.97.3.1262-1271.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann C., Davis B. D. Phenotypic suppression in Escherichia coli by chloramphenicol and other reversible inhibitors of the ribosome. J Bacteriol. 1969 Apr;98(1):152–159. doi: 10.1128/jb.98.1.152-159.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Endo H., Ohnishi Y. Mutations to spectinomycin resistance which alleviate the restriction of an amber suppressor by streptomycin resistance. J Bacteriol. 1969 Feb;97(2):940–943. doi: 10.1128/jb.97.2.940-943.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG E. M., CAVALLI-SFORZA L., LEDERBERG J. INTERACTION OF STREPTOMYCIN AND A SUPPRESSOR FOR GALACTOSE FERMENTATION IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Apr;51:678–682. doi: 10.1073/pnas.51.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. Streptomycin resistance; a genetically recessive mutation. J Bacteriol. 1951 May;61(5):549–550. doi: 10.1128/jb.61.5.549-550.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LEWIS C., CLAPP H. W. Actinospectacin, a new antibiotic. III. In vitro and in vivo evaluation. Antibiot Chemother (Northfield) 1961 Feb;11:127–133. [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MASON D. J., DIETZ A., SMITH R. M. Actinospectacin, a new antibiotic. I. Discovery and biological properties. Antibiot Chemother (Northfield) 1961 Feb;11:118–122. [PubMed] [Google Scholar]
- Mayuga C., Meier D., Wang T. Escherichia coli: the K 12 ribosomal protein and the streptomycin region of the chromosome. Biochem Biophys Res Commun. 1968 Oct 24;33(2):203–206. doi: 10.1016/0006-291x(68)90768-7. [DOI] [PubMed] [Google Scholar]
- NATHANS D., NOTANI G., SCHWARTZ J. H., ZINDER N. D. Biosynthesis of the coat protein of coliphage f2 by E. coli extracts. Proc Natl Acad Sci U S A. 1962 Aug;48:1424–1431. doi: 10.1073/pnas.48.8.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine T. F., Jr, Finland M. Observations on Bacteria Sensitive to, Resistant to, and Dependent upon Streptomycin. J Bacteriol. 1948 Aug;56(2):207–218. doi: 10.1128/jb.56.2.207-218.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- SPEYER J. F., LENGYEL P., BASILIO C. Ribosomal localization of streptomycin sensitivity. Proc Natl Acad Sci U S A. 1962 Apr 15;48:684–686. doi: 10.1073/pnas.48.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H. R-factor-mediated resistance to new aminoglycoside antibiotics. Lancet. 1967 Feb 4;1(7484):252–254. doi: 10.1016/s0140-6736(67)91309-8. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Transductional studies of thiamine and nicotinic acid requiring streptomycin resistant mutants of Salmonella typhimurium. J Gen Microbiol. 1960 Feb;22:102–112. doi: 10.1099/00221287-22-1-102. [DOI] [PubMed] [Google Scholar]
- WATANABE T., WATANABE M. Transduction of streptomycin resistance in Salmonella typhimurium. J Gen Microbiol. 1959 Aug;21:16–29. doi: 10.1099/00221287-21-1-16. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]



