Abstract
Several inositol-containing compounds play key roles in receptor-mediated cell signaling events. Here, we describe a function for a specific inositol polyphosphate, d-myo-inositol 1,4,5,6-tetrakisphosphate [Ins(1,4,5,6)P4], that is produced acutely in response to a receptor-independent process. Thus, infection of intestinal epithelial cells with the enteric pathogen Salmonella, but not with other invasive bacteria, induced a multifold increase in Ins(1,4,5,6)P4 levels. To define a specific function of Ins(1,4,5,6)P4, a membrane-permeant, hydrolyzable ester was used to deliver it to the intracellular compartment, where it antagonized epidermal growth factor (EGF)-induced inhibition of calcium-mediated chloride (Cl−) secretion (CaMCS) in intestinal epithelia. This EGF function is likely mediated through a phosphoinositide 3-kinase (PtdIns3K)-dependent mechanism because the EGF effects are abolished by wortmannin, and three different membrane-permeant esters of the PtdIns3K product phosphatidylinositol 3,4,5-trisphosphate mimicked the EGF effect on CaMCS. We further demonstrate that Ins(1,4,5,6)P4 antagonized EGF signaling downstream of PtdIns3K because Ins(1,4,5,6)P4 interfered with the PtdInsP3 effect on CaMCS without affecting PtdIns3K activity. Thus, elevation of Ins(1,4,5,6)P4 in Salmonella-infected epithelia may promote Cl− flux by antagonizing EGF inhibition mediated through PtdIns3K and PtdInsP3.
Inositol-based compounds are central to many signal transduction pathways. For example, phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] is a precursor for the phospholipase C product inositol 1,4,5-trisphosphate, which is a key regulator of intracellular calcium levels (1). Moreover, PtdIns(4,5)P2 is a substrate for phosphoinositide 3-kinase (PtdIns3K), which yields phosphatidylinositol 3,4,5-trisphosphate (PtdInsP3). PtdIns3K is triggered by a wide range of growth factors and cytokines, and it and/or its products contribute to the regulation of many cell functions, including cell proliferation, differentiation, and apoptosis (2). However, although more than 30 inositol-containing phospholipids and inositol polyphosphates have been identified, relatively little is known about the roles of most of these compounds in cellular signaling.
Chloride (Cl−) secretion by polarized epithelia at mucosal surfaces plays a key role in controlling salt and fluid secretion. In the intestine, for example, constitutive Cl− flux is important in the maintenance of normal hydration and provides solvent for extracellular solutes. Furthermore, Cl− and consequently water flux increase after infection with many enteric pathogens (e.g., Salmonella), and this host response contributes to the removal of the invading pathogen from the intestinal tract (3, 4). Several pathways have been identified that stimulate Cl− secretion through the elevation of either cAMP or intracellular calcium levels, which act on separate apically located Cl− channels (5). In addition, Cl− secretion is controlled by negative regulatory mechanisms. For example, we have identified two separate receptor-activated signaling cascades that inhibit the calcium-mediated Cl− secretory pathway [e.g., calcium-mediated Cl− secretion (CaMCS)] in intestinal epithelia: (i) muscarinic stimulation of inositol 3,4,5,6-tetrakisphosphate [Ins(3,4,5,6)P4] levels results in inhibition of CaMCS through a direct effect on Cl− flux (6–8) and (ii) epidermal growth factor (EGF) inhibits CaMCS through a PtdIns3K pathway (9, 10). Thus, the overall level of Cl− secretion is determined by a balance between stimulatory and inhibitory factors that act on the intestinal epithelium.
Invasive enteric bacteria enter and penetrate the intestinal epithelium to gain access to the underlying mucosa and initiate systemic infection. Bacterial entry into epithelial cells is an interactive process that requires bacterial and host cell signaling events (11, 12) and triggers epithelial cell responses that play a role in host protection against the invading bacteria (4, 13). A possible role for inositol polyphosphates in the epithelial response to bacterial invasion was suggested by earlier studies, in which Salmonella invasion elicited an overall increase in inositol polyphosphate turnover (14), but the nature and source of these changes remained undefined. Because inositol polyphosphates are potentially important in cell signaling, we examined the changes in the levels of specific cellular inositol polyphosphates in response to Salmonella invasion of intestinal epithelial cells. The data show that infection of intestinal epithelial cells with Salmonella, but not other invasive bacteria, induces Ins(1,4,5,6)P4 production. Furthermore, this inositol isomer has a function in epithelial cells by antagonizing signaling through PtdIns3K pathways. As a consequence, the negative regulation of CaMCS by EGF is antagonized, and could thereby contribute to the bacterially induced diarrhea.
MATERIALS AND METHODS
Reagents.
Inositol phosphates and their corresponding membrane-permeant acetoxymethyl esters were synthesized as described (6). Pluronic F-127 was a generous gift from BASF Bioresearch (Leverkusen, Germany). Membrane-permeant analogues of PtdInsP3 were prepared by total synthesis as will be described elsewhere (T.J., G. Sweeney, A. Klip, A.E.T.-K., and R.T., and C.S., M.T.R., H. H. Gillandt, and A.E.T.-K., unpublished work). The preparation of DiC16-BtPtdInsP3/AM was ≈5% DiC16-BtPtdInsP3/AM and 95% dipalmitoylglycerol whereas the preparations of DiC8-PtdInsP3/AM and DiC12-PtdInsP3/AM were >99% pure, as determined by proton nuclear magnetic resonance spectroscopy and positive ion mode electron spray mass spectroscopy. Radiolabeled inositol phosphates used as standards for HPLC were from NEN. Phosphatidylinositol for in vitro PtdIns3K assays was obtained from Avanti Polar Lipids. EGF was from Genzyme.
Cells.
The T84 human colon epithelial cell line (15) was maintained in 50% DMEM and 50% Ham’s F-12 medium, supplemented with 5% newborn calf serum and 2 mM glutamine. The LS174T human colon adenocarcinoma cell line (CL 188) was obtained from the American Type Culture Collection and maintained in DMEM supplemented with 10% fetal calf serum, 10 mM Hepes, and 2 mM glutamine.
Bacterial Strains.
The following bacteria were used in these studies (13, 16): Salmonella dublin, Salmonella typhi BRD691 (an aroA/aroC mutant that invades epithelial cells normally but does not replicate inside the cells), Yersinia enterocolitica, enteroinvasive Escherichia coli (serotype O29:NM), enterohemorrhagic E. coli (serotype O157), Shigella dysenteriae, Shigella flexneri, and nonpathogenic E. coli DH5α (GIBCO/BRL). Bacteria were grown and prepared for infection as described before (13, 16).
[3H]Inositol Labeling and Extraction of Cellular Inositol Polyphosphates.
Confluent epithelial monolayers in 6-well plates were incubated for 4 days with 50 μCi/well [3H]inositol (specific activity 80–120 Ci/mmol; Amersham) in inositol-free 50% DMEM, 50% Ham’s F-12 medium, supplemented with 5% dialyzed newborn calf serum. Cultures were washed three times with prewarmed medium (50% DMEM/50% Ham’s F-12 medium/1 mg/ml BSA) and infected with 5 × 108 bacteria/well using the same medium for various periods of time. Cultures were washed twice with ice-cold PBS and lysed for 5 min on ice in 0.5 ml/well 10% trichloroacetic acid/10 mM phytic acid, and extracts were neutralized using 1,1,2-trichlorotrifluoroethane/tri-n-octylamine. [3H]inositol polyphosphates in the extracts were separated on an Adsorbosphere SAX column (Alltech Associates), and the radioactive peaks were quantitated as described before (17).
Enantiomeric Identification of Ins(1,4,5,6)P4.
Cell extracts were fractionated by HPLC using an Adsorbosphere SAX column. The [3H]Ins(3,4,5,6)P4/[3H]Ins(1,4,5,6)P4 peak was separated from all other [3H]InsP4 isomers, desalted, and then incubated with partially purified Ins(1,4,5,6)P4 3-kinase with an internal standard of [33P]Ins(1,4,5,6)P4 (6). Rat hepatic Ins(1,4,5,6)P4 3-kinase was purified 240-fold, with a yield of 3%, by Mono Q (Pharmacia) anion-exchange chromatography (18) followed by blue A affinity chromatography and Ins(1,4,5)P3 affinity chromatography (A. Craxton, M. Hirata, and S.B.S., unpublished data). The enzyme preparation did not phosphorylate Ins(3,4,5,6)P4. The [33P]Ins(1,4,5,6)P4 standard was prepared by dephosphorylation of [33P]Ins(1,3,4,5,6)P5 by using multiple inositol polyphosphate phosphatase (19). [33P]Ins(1,3,4,5,6)P5 was purified from turkey erythrocytes that were labeled radioactively by using methods described before except that [33P]Pi was used instead of [32P]Pi (20). Equal amounts of 3H and 33P radioactivity were added to the reaction, and the ratio of [3H]Ins(3,4,5,6)P4:[3H]Ins(1,4,5,6)P4 in the original peak was determined by comparing the relative amounts of 3H- and 33P-labeled Ins(1,3,4,5,6)P5 formed. As an example, in a representative experiment, 2,263 dpm of the purified [3H]Ins(3,4,5,6)P4/[3H]Ins(1,4,5,6)P4 peak obtained from S. dublin-infected T84 cells was mixed with 1,675 dpm of [33P]Ins(1,4,5,6)P4 standard, and the appropriate buffer and Ins(1,4,5,6)P4 3-kinase were added. After 30 min, the reaction was found to contain 1,842 dpm [3H]Ins(1,3,4,5,6)P5 and 1,458 dpm [33P]Ins(1,3,4,5,6)P5. Thus, phosphorylation efficiencies were 1,842 dpm/2,263 dpm × 100% = 81% for the [3H]Ins(3,4,5,6)P4/[3H]Ins(1,4,5,6)P4 fraction and 1,458 dpm/1,675 dpm × 100% = 87% for the internal standard. It follows that 81%/87% × 100% = 93% of the [3H]Ins(3,4,5,6)P4/[3H]Ins(1,4,5,6)P4 peak was [3H]Ins(1,4,5,6)P4, with the remainder being [3H]Ins(3,4,5,6)P4.
Ussing Chamber Experiments.
T84 cells were seeded onto microporous filter inserts (Snapwells, Costar) and grown to confluence for 7–12 days. The inserts were mounted into modified Ussing chambers (Physiologic Instruments, San Diego), bathed with Ringer’s solution warmed to 37°C and gassed continuously with 95% O2/5% CO2 at a rate of 30–35 ml/min. The spontaneous potential difference across the monolayer was short-circuited with a voltage clamp (model VCC MC6, Physiologic Instruments). Short circuit current (Isc) and conductance were recorded at 4-s intervals by using acquire and analyse software 1.1 (Physiologic Instruments). Increased Isc stimulated through cholinergic pathways (e.g., after carbachol addition) in T84 cells is wholly reflective of Cl− secretion (21). [Ca2+]i levels were elevated with carbachol (100 μM) applied to the medium bathing the basolateral side of the monolayers.
PtdIns3K Assays.
PtdIns3K activity was measured in anti-PtdIns3K (rabbit polyclonal anti-85K subunit, Upstate Biotechnology, Lake Placid, NY) or mouse monoclonal anti-phosphotyrosine (clone 4G10, Upstate Biotechnology) immunoprecipitates exactly as described (10). For experiments to determine the effect of Ins(1,4,5,6)P4 on PtdIns3K activity in vitro, T84 cells were stimulated with EGF (16.3 nM) for 1 and 5 min, and PtdIns3K was immunoprecipitated and resuspended in reaction buffer (10). Ins(1,4,5,6)P4 (200 μM) was added 10 min before addition of [γ-32P]ATP. Wortmannin (50 nM) was used as a control.
RESULTS
Salmonella Infection Induces Ins(1,4,5,6)P4 Production in Intestinal Epithelial Cells.
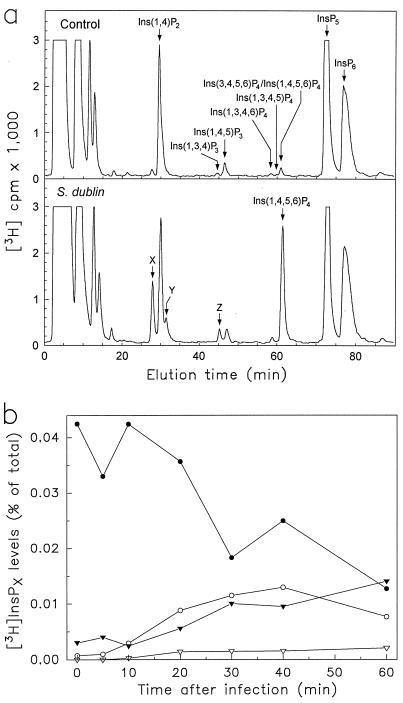
To determine changes in inositol polyphosphate metabolism after Salmonella infection, monolayers of T84 human colonic epithelial cells were labeled with [3H]inositol and infected for varying periods of time with S. dublin. Inositol polyphosphates were extracted and resolved by HPLC equipped with an on-line radioactivity detector. During the 60-min period immediately after infection, there were no significant changes in the levels of InsP6 or inositol polyphosphates that typically accumulate when phospholipase C is activated, such as [3H]Ins(1,4,5)P3, [3H]Ins(1,3,4,5)P4, and [3H]Ins(1, 4)P2 (Fig. 1a). In contrast, levels of [3H]Ins(3,4,5,6)P4/[3H]Ins(1,4,5,6)P4 (enantiomers that cannot be resolved by HPLC) increased within 10 min after S. dublin infection, reaching a 14-fold maximum 30–40 min after infection (Fig. 1). After enantiomeric analysis, this peak was found to consist primarily of Ins(1,4,5,6)P4 (Fig. 1). We estimate that the intracellular concentrations of Ins(1,4,5,6)P4 and Ins(3,4,5,6)P4 were 4.7 and 0.9 μM, respectively, 30 min after S. dublin infection, based on our previous determinations of the resting levels of Ins(1,4,5,6)P4 and Ins(3,4,5,6)P4 in T84 cells (6) and the relative increase of these enantiomers after infection. Levels of Ins(1,4,5,6)P4 decreased slowly after 40 min and returned to near baseline by 3 h after infection (Table 1). The elevation in [3H]Ins(1,4,5,6)P4 levels was accompanied by a parallel decline in [3H]InsP5 levels, reaching a minimum of 50% of control levels 30–60 min after infection (Fig. 1b) and by smaller increases in an InsP3 isomer (labeled Z in Fig. 1a) and two InsP2 isomers (labeled X and Y in Fig. 1a). Compounds X, Y, and Z did not match elution patterns of available inositol phosphate standards. Similar observations were made using another human intestinal epithelial cell line, LS174T, in which [3H]Ins(1,4,5,6)P4 levels increased 11.3-fold after S. dublin infection, indicating that the changes in cellular inositol polyphosphates after infection represent a general response of epithelial cells.
Figure 1.
HPLC analysis of inositol polyphosphate levels after S. dublin infection of T84 human colonic epithelial cells. [3H]Inositol-labeled T84 monolayers were infected with S. dublin, and inositol polyphosphates were extracted and analyzed by an HPLC system that resolves three InsP4 peaks, as demonstrated (17). (a) A representative radiochromatogram from control cells (top) and cells infected for 30 min with S. dublin (bottom). Arrows indicate elution times of known inositol phosphate standards. The Ins(3,4,5,6)P4/Ins(1,4,5,6)P4 peak was collected and analyzed as described in Materials and Methods and found to consist of 84 ± 5% (mean ± SEM, n = 3) Ins(1,4,5,6)P4 after S. dublin infection, with the remainder being Ins(3,4,5,6)P4. In unstimulated cells, the same peak contained approximately equal amounts of both isomers (data not shown) (6). (b) A time course of the levels of InsP5 (•), Ins(1,4,5,6)P4 (○), InsP3-Z (▿), and InsP2-X and Y (▾). Data are displayed as the fraction of radioactivity associated with the specific InsPx relative to the total radioactivity in all cellular InsPx.
Table 1.
Increase in Ins(1,4,5,6)P4 levels after Salmonella infection of T84 intestinal epithelial cells
| Bacteria added | Time after infection, min | [3H]Ins(1,4,5,6)P4 levels, ratio infected/control | n |
|---|---|---|---|
| S. dublin lane | 30 | 13.9 ± 0.8 | 4 |
| S. dublin lane | 60 | 10.3 ± 1.3 | 5 |
| S. dublin lane | 120 | 3.6 | 2 |
| S. dublin lane | 180 | 1.9 | 2 |
| S. typhi BRD691 | 30 | 10.5 | 2 |
| S. dublin SB133 (invA) | 30 | 1.9 ± 0.1 | 3 |
| S. flexneri | 60 | 1.9 | 2 |
| S. dysenteriae | 60 | 2.3 | 2 |
| Y. enterocolitica | 60 | 2.6 ± 0.1 | 3 |
| E. coli O29:NM | 60 | 2.0 ± 0.1 | 5 |
| E. coli O157 | 60 | 2.3 ± 0.2 | 4 |
| E. coli DH5α | 60 | 2.1 ± 0.2 | 4 |
| LPS | 60 | 1.0 | 2 |
[3H]Inositol-labeled T84 monolayers were infected with various bacteria for the indicated times. [3H]Ins(1,4,5,6)P4 levels were determined by HPLC and are expressed as ratio of the level in infected cells to that in control cells. Results are means ± SEM of the number of determinations indicated in the last column. LPS (lipopolysaccharide) from E. coli O111 was used at 10 μg/ml.
Infection of T84 cells with another invasive Salmonella strain, S. typhi BRD691 (16), also increased [3H]Ins(1,4,5,6)P4 levels (Table 1). In contrast, a mutant strain of S. dublin, SB133, which attaches normally to epithelial cells but does not invade them (22), increased [3H]Ins(1,4,5,6)P4 levels only minimally, indicating that invasion of host cells by Salmonella was required for this response. However, bacterial invasion alone was not sufficient to increase Ins(1,4,5,6)P4 levels because infection of T84 cells with several other invasive Gram-negative bacteria, including S. flexneri, S. dysenteriae, Y. enterocolitica, and enteroinvasive E. coli (serotype O29:NM), caused only small increases in [3H]Ins(1,4,5,6)P4 levels (Table 1). Furthermore, addition to T84 monolayers of noninvasive Gram-negative bacteria such as enterohemorrhagic E. coli (serotype O157) or a nonpathogenic E. coli (strain DH5α) or addition of bacterial lipopolysaccharide, also had little to no effect on [3H]Ins(1,4,5,6)P4 levels (Table 1).
Ins(1,4,5,6)P4 Inhibits EGF-Induced Inhibition of Calcium-Mediated Cl− Secretion in Intestinal Epithelial Cells.
We next investigated the functional relationship between the Salmonella-induced changes in Ins(1,4,5,6)P4 levels and the secretory response to the infection. Such studies in bacteria-infected cells are hampered by the asynchronous nature of the infection with Salmonella and by the fact that a variable proportion of epithelial cells in tissue culture become infected. In addition, Salmonella infection is likely to have other effects on epithelial cells that would complicate the identification of a role for Ins(1,4,5,6)P4. To circumvent these problems, we raised Ins(1,4,5,6)P4 levels in resting T84 cells by using membrane-permeant d-2,3-di-O-butyryl-myo-inositol 1,4,5,6-tetrakisphosphate octakis(acetoxymethyl) ester (Bt2Ins(1,4,5,6)P4/AM), which diffuses into epithelial cells and subsequently is hydrolyzed by ubiquitous, nonspecific cellular esterases to release Ins(1,4,5,6)P4 (6). As a functional assay for these experiments, we assessed the effect of Ins(1,4,5,6)P4 on transepithelial Cl− secretion across polarized T84 monolayers mounted in Ussing chambers.
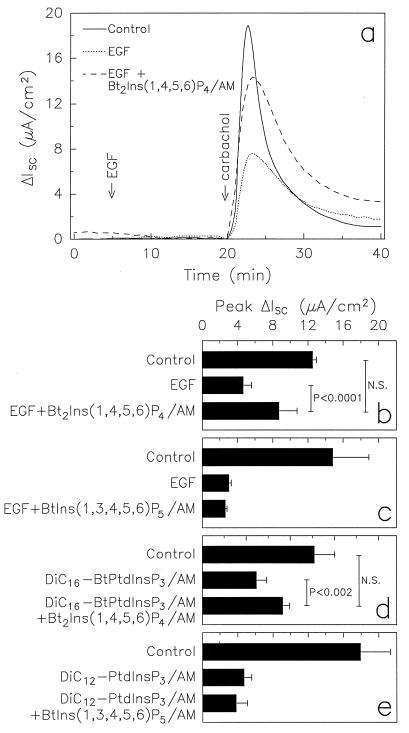
We found previously that Bt2Ins(1,4,5,6)P4/AM had no effect on Cl− secretion by itself, nor did it affect [Ca2+]i levels (6). Furthermore, Bt2Ins(1,4,5,6)P4/AM neither potentiated nor inhibited the effects of known agonists of Cl− secretion. For example, thapsigargin stimulates increased Cl− secretion through an increase in [Ca2+]i levels (i.e., CaMCS), and neither Cl− secretion nor [Ca2+]i levels are affected by 400 μM of Bt2Ins(1,4,5,6)P4/AM pretreatment (6). We therefore questioned whether Ins(1,4,5,6)P4 might up-regulate Cl− secretion by antagonizing a process that normally constrains transepithelial Cl− flux. For example, Ins(3,4,5,6)P4 inhibits CaMCS (6). However, Ins(1,4,5,6)P4 did not affect this function of Ins(3,4,5,6)P4 (6). We therefore turned our attention to EGF-mediated inhibition of CaMCS (9). Addition of Bt2Ins(1,4,5,6)P4/AM to T84 monolayers significantly reversed inhibition of CaMCS that was induced by EGF preincubation (Fig. 2 a and b). In contrast, this inhibitory EGF function was not attenuated by addition of membrane-permeant esters of Ins(1,3,4,5,6)P5 (Fig. 2c) or Ins(3,4,5,6)P4 (data not shown), the latter being the enantiomer of Ins(1,4,5,6)P4. These findings demonstrate that Ins(1,4,5,6)P4 can, in an enantiomerically specific manner, up-regulate CaMCS by reversing EGF-mediated inhibition of this process. Furthermore, the Ins(1,4,5,6)P4 action was specific for CaMCS because addition of Bt2Ins(1,4,5,6)P4/AM to T84 monolayers did not reverse EGF inhibition of cAMP-mediated Cl− secretion (data not shown).
Figure 2.
Membrane-permeant Ins(1,4,5,6)P4 reverses EGF- and PtdInsP3-mediated inhibition of CaMCS. T84 monolayers were incubated for 30 min with the indicated membrane-permeant esters (200 μM) or vehicle (dimethyl sulfoxide/5% Pluronic) in Ringer’s solution before mounting in Ussing chambers. Based on our previous findings that 1–2% of the membrane-permeant inositol polyphosphate esters enter T84 cells (6), we estimate that the intracellular concentration of Ins(1,4,5,6)P4 was ≈2–4 μM after 30 min of incubation with its membrane-permeant ester. (a) Time course of ΔIsc. EGF (16.3 nM) was added basolaterally, followed 15 min later by carbachol (100 μM) to acutely elevate [Ca2+]i levels. Controls also were stimulated with carbachol but not pretreated with EGF. Data are means of duplicate measurements from one representative of three experiments. (b-e) Peak ΔIsc after carbachol addition. Data are means ± SEM of 4–10 experiments. Statistical significance was determined by Student’s t test (N.S., not significant). (d) DiC16-BtPtdInsP3/AM was used at 100 μM. Similar results were obtained with DiC8-PtdInsP3/AM (ΔIsc in μA/cm2; controls 14.0 ± 1.9; 200 μM DiC8-PtdInsP3/AM 7.3 ± 1.7; DiC8-PtdInsP3 plus Bt2Ins(1,4,5,6)P4/AM 9.9 ± 1.8; n = 7–8). Not shown, treatment of T84 cells with Bt2Ins(1,4,5,6)P4/AM, BtIns(1,3,4,5,6)P5/AM, or up to 10 mM dipalmitoylglycerol (which was a contaminant in the DiC16-BtPtdInsP3/AM preparations) did not alter peak ΔIsc after carbachol addition compared with control cells.
Ins(1,4,5,6)P4 Antagonizes Phosphoinositide 3-Kinase Signaling Pathways in Epithelial Cells.
The Ins(1,4,5,6)P4 effect on EGF-mediated inhibition of CaMCS is similar to that reported for the PtdIns3K inhibitor wortmannin (10). This raised the possibility that Ins(1,4,5,6)P4 might interact with PtdIns3K signaling pathways. We first investigated whether PtdIns3K activity itself was affected by Ins(1,4,5,6)P4. However, addition of up to 200 μM Ins(1,4,5,6)P4 had no effect on the activity of immunoprecipitated PtdIns3K in vitro (data not shown). Furthermore, Ins(1,4,5,6)P4 did not inhibit PtdIns3K activation after EGF stimulation of T84 cells because assays of PtdIns3K activity (see Materials and Methods) showed that EGF stimulated this activity in Bt2Ins(1,4,5,6)P4/AM-treated cells as much, or more, than it did in control cells [the ratio of PtdIns3K activity in EGF-stimulated relative to basal activity in unstimulated control cells was 3.3-fold in Bt2Ins(1,4,5,6)P4/AM-pretreated cells and 2.7-fold in untreated cells (n = 2); these values fall within the range of those reported (10)].
We next tested whether Ins(1,4,5,6)P4 acted downstream of PtdIns3K. PtdInsP3 is a major product of PtdIns3K in EGF-stimulated T84 cells, and our previous studies suggested that PtdInsP3, or one of its metabolites, mediated the EGF effects on CaMCS (10). To test this hypothesis directly, three membrane-permeant analogues of PtdInsP3 were synthesized: dipalmitoyl dl-6-O-butyryl-PtdIns(3, 4, 5)P3/AM (DiC16-BtPtdInsP3/AM), dioctanoyl d-PtdInsP3/AM (DiC8-PtdInsP3/AM), and dilauryl d-PtdInsP3/AM (DiC12-PtdInsP3/AM). The three different analogues of PtdInsP3 have different solubilities and were produced through separate synthetic pathways (T.J., G. Sweeney, A. Klip, A.E.T.-K., and R.T., and C.S., M.T.R., H. H. Gillandt, and A.E.T.-K., unpublished work). Preincubation of T84 monolayers with 100–200 μM of any of the three membrane-permeant forms of PtdInsP3, but not with PtdInsP3 itself (which is not expected to enter cells), inhibited CaMCS by up to 74% (Fig. 2 d and e) but had no effect on [Ca2+]i levels after carbachol stimulation (T.J., G. Sweeney, A. Klip, A.E.T.-K., and R.T., unpublished work). Inhibition of CaMCS by DiC12-PtdInsP3/AM was dose-dependent, with 38.5 ± 3.8% inhibition (mean ± SEM, n = 4) after incubation with 20 μM of the ester and 17% inhibition (mean, n = 2) after incubation with 2 μM ester. The maximal inhibition of CaMCS by the membrane-permeant forms of PtdInsP3 was comparable to that observed after EGF stimulation of T84 cells (9). Furthermore, addition of EGF to monolayers preincubated with DiC12-PtdInsP3/AM did not result in additional inhibition of CaMCS (data not shown), indicating that PtdInsP3 and EGF work through the same mechanism. We then tested if co-incubation of monolayers with Bt2Ins(1,4,5,6)P4/AM and either DiC16-BtPtdInsP3/AM or DiC8-PtdInsP3/AM would reverse the effect of membrane-permeant PtdInsP3 on CaMCS. As shown in Fig. 2d, preincubation of T84 monolayers with membrane-permeant forms of both Ins(1,4,5,6)P4 and PtdInsP3 led to significantly less inhibition of CaMCS than preincubation with membrane-permeant PtdInsP3 alone. In contrast, preincubation with a membrane-permeant form of Ins(1,3,4,5,6)P5 did not affect inhibition of CaMCS by PtdInsP3 (Fig. 2e).
DISCUSSION
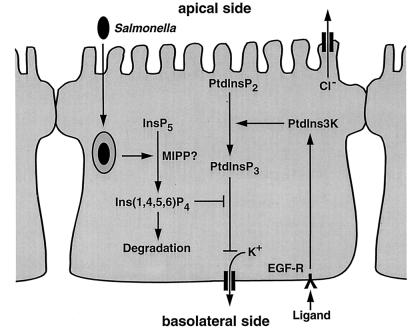
These findings represent the first evidence that a physiologically relevant stimulus, the entry of Salmonella into colonic epithelia, elicits the elevation of Ins(1,4,5,6)P4 levels. The changes in inositol polyphosphates after Salmonella entry contrast markedly with those observed after receptor-mediated phospholipase C activation, such as increases in Ins(1,4,5)P3, Ins(1,3,4,5)P4, Ins(1,3,4)P3, Ins(1,3,4,6)P4, and Ins(1,4)P2 (17), which suggests that the effects of Salmonella invasion on inositol phosphate levels are independent of phospholipase C. Furthermore, elevated Ins(1,4,5,6)P4 levels have been attributed to constitutive src activation in transformed fibroblasts (23), but tyrosine kinases were not involved in the Salmonella-induced Ins(1,4,5,6)P4 increase because specific tyrosine kinase inhibitors (genistein, herbimycin A, and tyrphostin A25) did not affect this cellular response (data not shown). Therefore, Salmonella appears to affect inositol phosphate metabolism through a unique mechanism. The fact that S. dublin did not alter InsP6 levels and the demonstration of reciprocal changes in the levels of InsP5 and Ins(1,4,5,6)P4, together suggest that S. dublin infection might activate a phosphatase, such as multiple inositol polyphosphate phosphatase (previously inositol polyphosphate 3-phosphatase) (19), to dephosphorylate InsP5 to Ins(1,4,5,6)P4 (as schematically depicted in Fig. 3, left).
Figure 3.
Proposed model of the mechanism of the effect of Salmonella-induced increase in Ins(1,4,5,6)P4 levels on ion transport and PtdIns3K signaling. CaMCS is accompanied by basolateral K+ efflux, which contributes to the driving force for Cl− secretion and can be limiting for Cl− secretion after elevation of [Ca2+]i levels. We have found that DiC12-PtdInsP3/AM inhibited K+ efflux (as measured by Rb+ efflux) in carbachol-stimulated epithelial monolayers (T.J., G. Sweeney, A. Klip, A.E.T.-K., and R.T., unpublished work). Thus, PtdInsP3-mediated inhibition of K+ efflux can suppress CaMCS. MIPP, multiple inositol polyphosphate phosphatase; EGF-R, EGF receptor. Relevant ligands for the EGF receptor in this model include EGF and transforming growth factor α. Arrowheads depict stimulatory, and capped lines designate inhibitory effects.
Our data suggest that Ins(1,4,5,6)P4 prevents EGF from inhibiting CaMCS in polarized epithelial cells by antagonizing a PtdInsP3 signaling pathway. Transforming growth factor α, which, like EGF, binds to and activates the EGF receptor, is constitutively expressed in intestinal epithelial cells (24) and inhibits Cl− secretion through a PtdIns3K pathway (data not shown). Tonic inhibition through this pathway could be counteracted by the Salmonella-induced increase in Ins(1,4,5,6)P4 (as schematically shown in Fig. 3, right). Such a mechanism would be expected to act early after infection and mostly in those epithelial cells that are infected with Salmonella and could result in diarrhea although other mechanisms also contribute to this outcome (3, 4, 25). Moreover, because Ins(1,4,5,6)P4 disinhibits CaMCS, this inositol polyphosphate, or an analogue, may be useful in treating cystic fibrosis, in which mucosal Cl− secretion is reduced and depends primarily on CaMCS (26). Because our data indicate that the effect of Ins(1,4,5,6)P4 on Cl− secretion was downstream of PtdIns3K, it is possible that Ins(1,4,5,6)P4 competes with PtdInsP3 for effector binding sites because Ins(1,4,5,6)P4 is a partial structural analog of the PtdInsP3 headgroup (27). As a result, Ins(1,4,5,6)P4 could compete with phosphoinositide binding to pleckstrin homology domains of signaling proteins and inhibit the normal recruitment of signal transduction complexes to the plasma membrane (28, 29).
Acknowledgments
This work was supported by grants from the National Institutes of Health, Universitywide AIDS Research Program, Fiterman Foundation, Deutsche Forschungsgemeinschaft, and the Crohn’s and Colitis Foundation of America.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PtdIns3K, phosphoinositide 3-kinase; PtdInsP3, phosphatidylinositol 3,4,5-trisphosphate; CaMCS, calcium-mediated chloride secretion; EGF, epidermal growth factor; Ins(1,4,5,6)P4, d-myo-inositol 1,4,5,6-tetrakisphosphate; ΔIsc, short circuit current.
References
- 1.Berridge M J. Am J Nephrol. 1997;17:1–11. doi: 10.1159/000169064. [DOI] [PubMed] [Google Scholar]
- 2.Toker A, Cantley L C. Nature (London) 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 3.Giannella R A, Rout W R, Formal S B. Infect Immun. 1977;17:136–139. doi: 10.1128/iai.17.1.136-139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckmann L, Stenson W F, Savidge T C, Lowe D C, Barrett K E, Fierer J, Smith J R, Kagnoff M F. J Clin Invest. 1997;100:296–309. doi: 10.1172/JCI119535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett K E. Am J Physiol. 1997;272:C1069–76. doi: 10.1152/ajpcell.1997.272.4.C1069. [DOI] [PubMed] [Google Scholar]
- 6.Vajanaphanich M, Schultz C, Rudolf M T, Wasserman M, Enyedi P, Craxton A, Shears S B, Tsien R Y, Barrett K E, Traynor-Kaplan A. Nature(London) 1994;371:711–714. doi: 10.1038/371711a0. [DOI] [PubMed] [Google Scholar]
- 7.Xie W, Kaetzel M A, Bruzik K S, Dedman J R, Shears S B, Nelson D J. J Biol Chem. 1996;271:14092–14097. doi: 10.1074/jbc.271.24.14092. [DOI] [PubMed] [Google Scholar]
- 8.Ismailov I I, Fuller C M, Berdiev B K, Shlyonsky V G, Benos D J, Barrett K E. Proc Natl Acad Sci USA. 1996;93:10505–10509. doi: 10.1073/pnas.93.19.10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uribe J M, Gelbmann C M, Traynor-Kaplan A E, Barrett K E. Am J Physiol. 1996;271:C914–922. doi: 10.1152/ajpcell.1996.271.3.C914. [DOI] [PubMed] [Google Scholar]
- 10.Uribe J M, Keely S J, Traynor-Kaplan A E, Barrett K E. J Biol Chem. 1996;271:26588–26595. doi: 10.1074/jbc.271.43.26588. [DOI] [PubMed] [Google Scholar]
- 11.Falkow S, Isberg R R, Portnoy D A. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 12.Galan J E, Bliska J B. Ann Rev Cell Dev Biol. 1996;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 13.Eckmann L, Kagnoff M F, Fierer J. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruschkowski S, Rosenshine I, Finlay B B. FEMS Microbiol Lett. 1992;74:121–126. doi: 10.1016/0378-1097(92)90416-l. [DOI] [PubMed] [Google Scholar]
- 15.Weymer A, Huott P, Liu W, McRoberts J A, Dharmsathaphorn K. J Clin Invest. 1985;76:1828–1836. doi: 10.1172/JCI112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang G T, Eckmann L, Savidge T C, Kagnoff M F. J Clin Invest. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachintorn U, Vajanaphanich M, Barrett K E, Traynor-Kaplan A E. Am J Physiol. 1993;264:C671–6. doi: 10.1152/ajpcell.1993.264.3.C671. [DOI] [PubMed] [Google Scholar]
- 18.Craxton A, Erneux C, Shears S B. J Biol Chem. 1994;269:4337–4342. [PubMed] [Google Scholar]
- 19.Craxton A, Ali N, Shears S B. Biochem J. 1995;305:491–498. doi: 10.1042/bj3050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens L R, Downes C P. Biochem J. 1990;265:435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dharmsathaphorn K, Cohn J, Beuerlein G. Am J Physiol. 1989;256:C1224–30. doi: 10.1152/ajpcell.1989.256.6.C1224. [DOI] [PubMed] [Google Scholar]
- 22.Galan J E, Curtiss R., 3 Infect Immun. 1991;59:2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattingly R R, Stephens L R, Irvine R F, Garrison J C. J Biol Chem. 1991;266:15144–15153. [PubMed] [Google Scholar]
- 24.Koyama S Y, Podolsky D K. J Clin Invest. 1989;83:1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dharmsathaphorn K, Traynor-Kaplan A. In: Textbook of Internal Medicine. Kelley W N, editor. Philadelphia: Lippincott; 1991. pp. 398–404. [Google Scholar]
- 26.Anderson M P, Welsh M J. Proc Natl Acad Sci USA. 1991;88:6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dijken P, de Haas J R, Craxton A, Erneux C, Shears S B, Van Haastert P J. J Biol Chem. 1995;270:29724–29731. doi: 10.1074/jbc.270.50.29724. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi H, Kanematsu T, Misumi Y, Yaakob H B, Yagisawa H, Ikehara Y, Watanabe Y, Tan Z, Shears S B, Hirata M. Biochem J. 1996;318:561–568. doi: 10.1042/bj3180561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemmon M A, Ferguson K M, Schlessinger J. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]