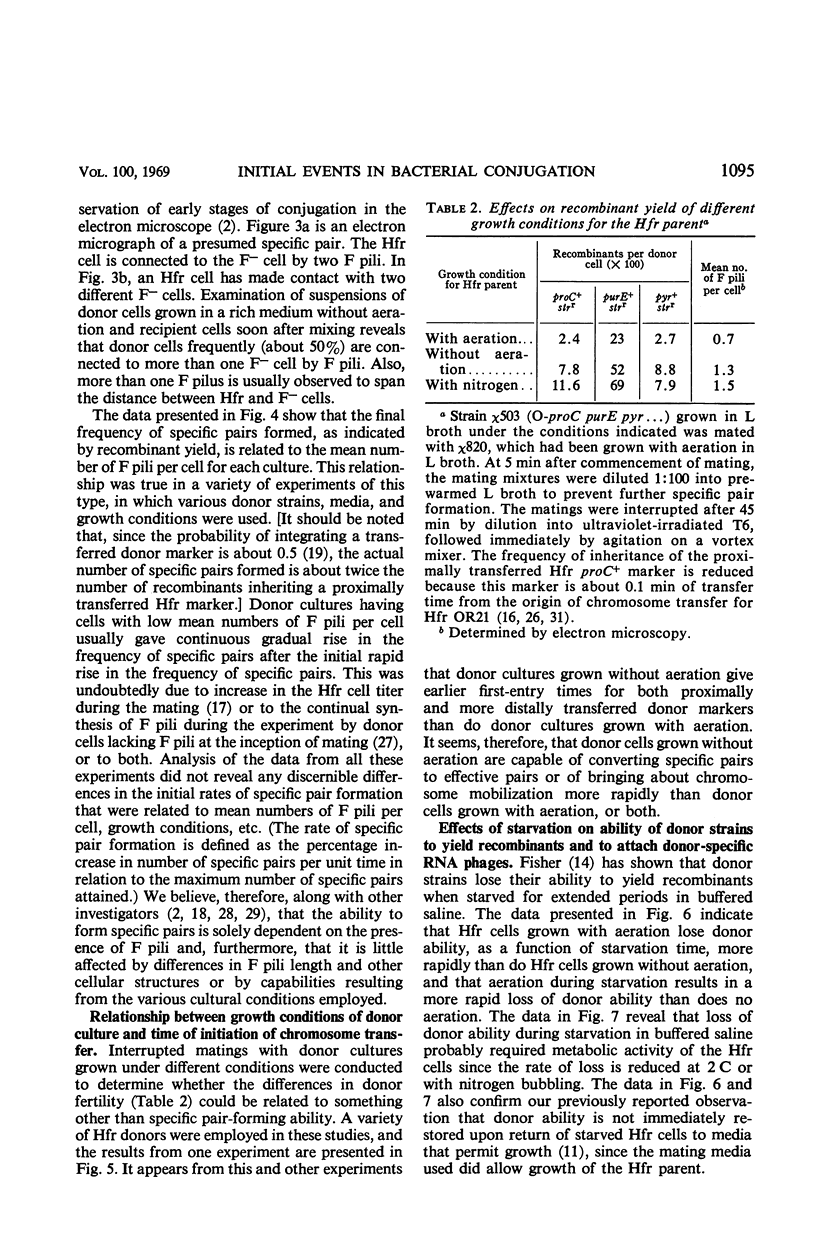
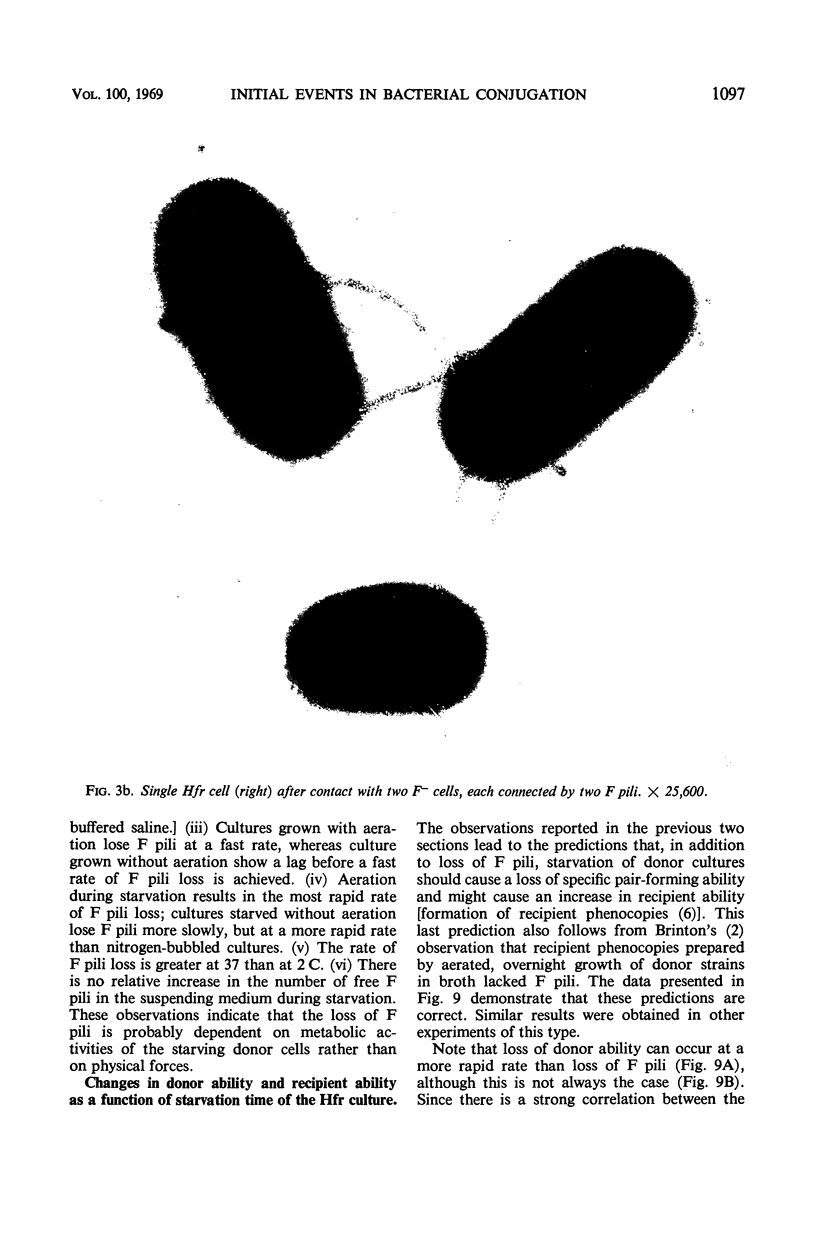
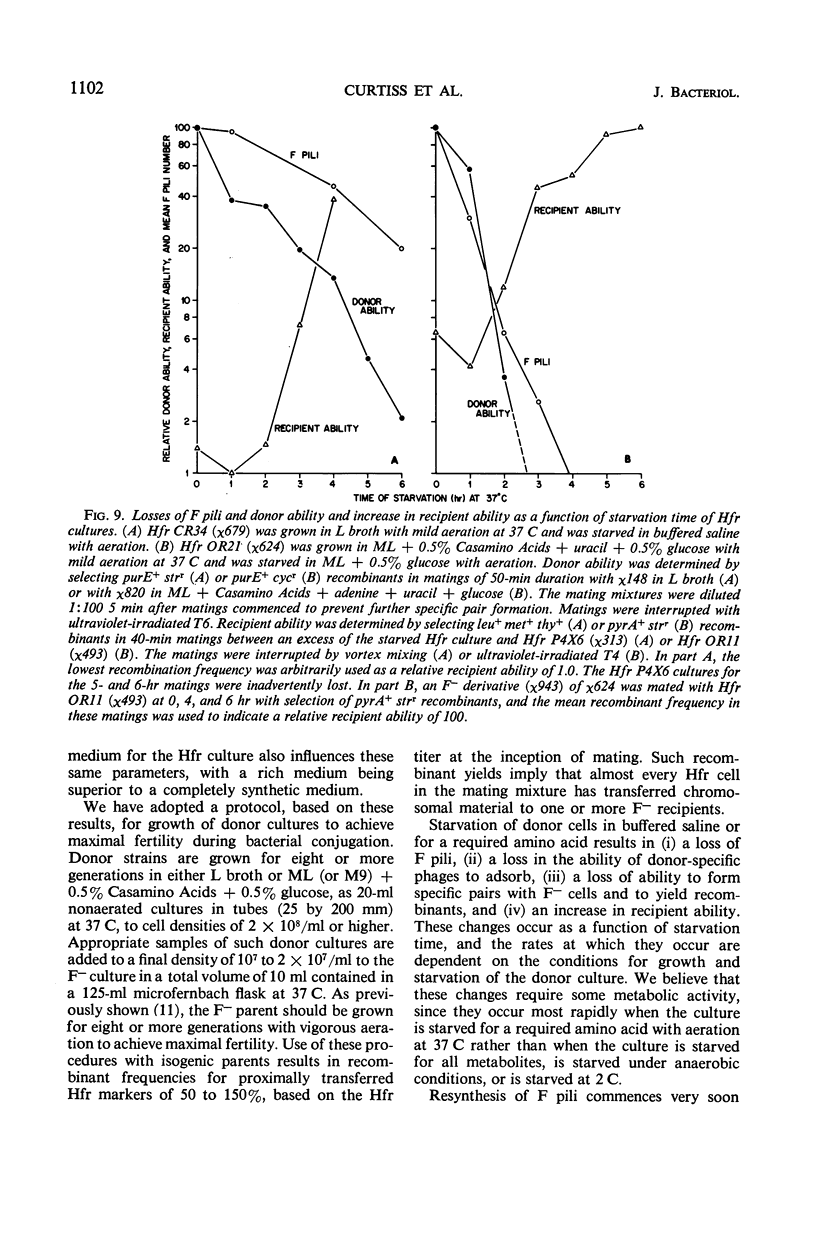
Abstract
We initiated these studies to learn more about the initial events during bacterial conjugation and to optimize conditions for their occurrence. We found that cells in donor cultures grown anaerobically prior to mating have (i) a higher mean number of F pili per cell, (ii) longer F pili, (iii) a higher probability of forming specific pairs with F− cells, and (iv) a faster rate of initiation of chromosome transfer than cells grown aerobically. The growth medium for the donor culture also influences these same parameters: a rich medium is superior to a completely synthetic medium. Starvation of donor cells in buffered saline or for a required amino acid results in (i) a loss of F pili, (ii) a loss in the ability of donor-specific phages to adsorb, (iii) a loss of ability to form specific pairs with F− cells and to yield recombinants, and (iv) an increase in recipient ability. These changes occur as a function of starvation time, and at rates which are dependent on the conditions of prior growth and starvation of the donor culture. Either treatment provides a rapid method for the production of F− phenocopies from donor cultures. Resynthesis of F pili by cells within a starved donor culture commences very soon after restoration of normal growth conditions, but full restoration of donor ability, as measured by recombinant yield, occurs at a slower rate. We found, along with other investigators, that F pili are essential for specific pair formation. We also found, however, that the presence of F pili is not sufficient for display of donor ability, nor is the absence of F pili enough for cells to exhibit recipient ability. This suggests, therefore, that one or more components, in addition to F pili, are necessary for the conversion of specific pairs to effective pairs (or for chromosome mobilization, or both) and for preventing donor cells from acting as recipients. On the basis of our results, we suggest optimal conditions for achieving high mating efficiencies.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- CAVALLI L. L., LEDERBERG J., LEDERBERG E. M. An infective factor controlling sex compatibility in Bacterium coli. J Gen Microbiol. 1953 Feb;8(1):89–103. doi: 10.1099/00221287-8-1-89. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Ultraviolet-induced genetic recombination in a partially diploid strain of Escherichia coli. Genetics. 1968 Jan;58(1):9–54. doi: 10.1093/genetics/58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER K. W. The nature of the endergonic processes in conjugation in Escherichia coli K-12. J Gen Microbiol. 1957 Feb;16(1):136–145. doi: 10.1099/00221287-16-1-136. [DOI] [PubMed] [Google Scholar]
- Fisher K. W. Amino acid deprivation and its effect on mating ability in Escherichia coli K12. Genet Res. 1966 Aug;8(1):115–118. doi: 10.1017/s0016672300009964. [DOI] [PubMed] [Google Scholar]
- Glansdorff N. Pseudoinversions in the chromosome of Escherichia coli K-12. Genetics. 1967 Jan;55(1):49–61. doi: 10.1093/genetics/55.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Caro L. G. DNA transfer in bacterial conjugation. J Mol Biol. 1966 Apr;16(2):269–284. doi: 10.1016/s0022-2836(66)80172-9. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The sex hair of E. coli as sensory fiber, conjugation tube, or mating arm? Biochem Biophys Res Commun. 1967 Jun 23;27(6):674–680. doi: 10.1016/s0006-291x(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Ishibashi M. F pilus as f+ antigen. J Bacteriol. 1967 Jan;93(1):379–389. doi: 10.1002/path.1700930144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle P. Evidence for the identity of the mating-specific site of male cells of Escherichia coli with the receptor site of an RNA phage. Biochem Biophys Res Commun. 1967 Apr 7;27(1):81–87. doi: 10.1016/s0006-291x(67)80043-3. [DOI] [PubMed] [Google Scholar]
- Krisch R. E., Kvetkas M. J. Inhibition of bacterial mating by amino acid deprivation. Biochem Biophys Res Commun. 1966 Mar 22;22(6):707–711. doi: 10.1016/0006-291x(66)90206-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOEB T. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science. 1960 Mar 25;131(3404):932–933. doi: 10.1126/science.131.3404.932. [DOI] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Raizen E., Knight W. S., Brinton C. C., Jr Functions of F pili in mating-pair formation and male bacteriophage infection studies by blending spectra and reappearance kinetics. J Bacteriol. 1969 Jun;98(3):1307–1319. doi: 10.1128/jb.98.3.1307-1319.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F. An antigen termed f-plus occurring in F-plus E. coli strains. Acta Pathol Microbiol Scand. 1960;48:37–46. [PubMed] [Google Scholar]
- Pittard J., Walker E. M. Conjugation in Escherichia coli: recombination events in terminal regions of transferred deoxyribonucleic acid. J Bacteriol. 1967 Nov;94(5):1656–1663. doi: 10.1128/jb.94.5.1656-1663.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]






