One goal of synthetic chemistry is to prepare a target molecule by a minimum number of transformations with high stereo-selectivity and efficiency. Despite significant advances made in recent years in the field of synthetic chemistry, preparation of many organic compounds still require multi-step chemical reactions. The lengthy operations can be tedious and time-consuming, and can suffer from low yields. However, as more biosynthetic pathways for natural products are characterized, the idea of exploiting these pathways to produce desired products has become increasingly attractive. It has recently been demonstrated that the biosynthetic machineries can be manipulated to produce tailor-made new compounds.1 Moreover, due to the high regio-and stereo-specificity of enzyme catalysis, the enzymatic synthesis may be carried out in one-pot, 2 which is not possible for most chemical reactions. Such an approach avoids the isolation and/or the accumulation of unstable intermediates, and also minimizes the use of chemicals and production of waste. The environmentally benign nature of these biosynthetic approaches is highly attractive.
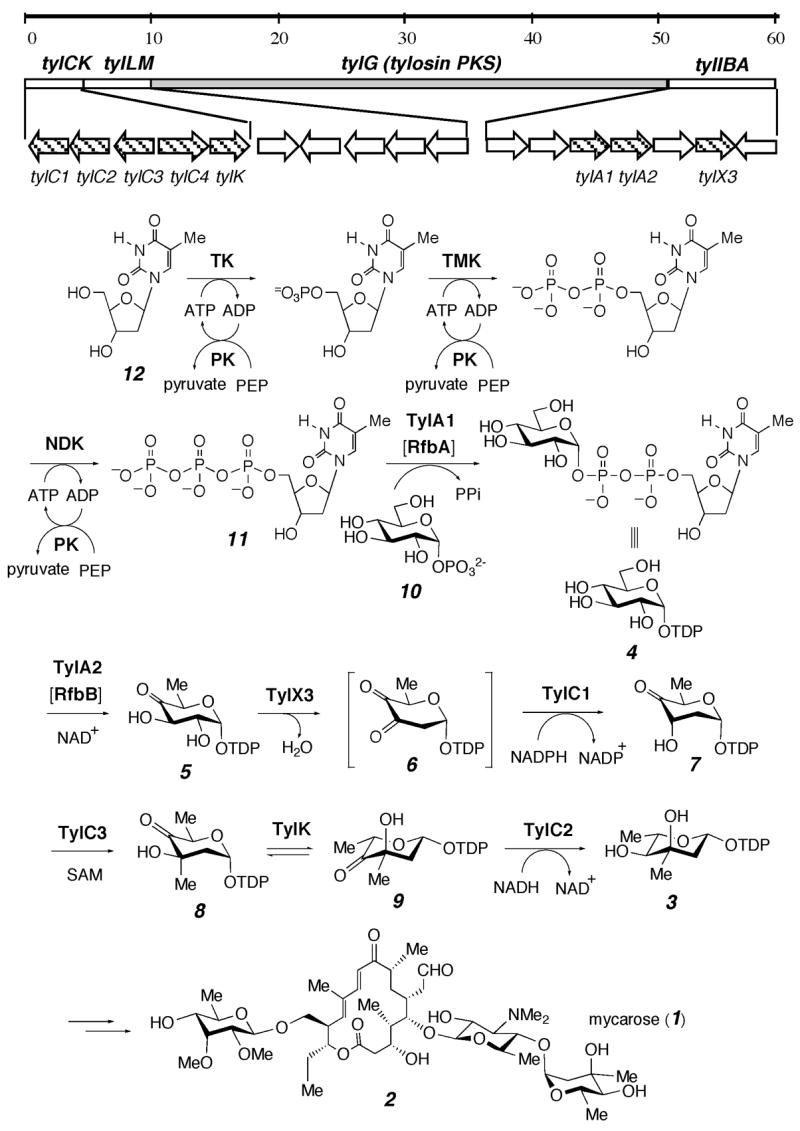
As a part of our effort to elucidate the biosynthetic pathways of unusual sugars, we have recently cloned and identified a number of sugar biosynthetic gene clusters, 3 including the entire pathway for the formation of mycarose (1).4 L-Mycarose is a key component of the macrolide antibiotic tylosin (2), which is produced by Streptomyces fradiae. It is also found in a few other clinically useful antibiotics. In order to generate new bioactive compounds by derivatization of structurally diverse macrolide aglycones with L-mycarose using selected glycosyltransferases, it is necessay to have large quantities of TDP-L-mycarose (3) readily accessible as it is the sugar substrate form recognized by the glycosyltransferases. Although L-mycarose has already been chemically synthesized, 5 attempts to prepare TDP-L-mycarose are hampered by the facile loss of the TDP substituent at C-1. To circumvent this problem, we explored a biosynthetic approach for the preparation of the compound.
As shown in Scheme 1, TDP-L-mycarose (3) is derived from TDP-D-glucose (4) via six enzymatic reactions.4 The key intermediate 7 is produced from 4 in three separate steps catalyzed by a 4,6-dehydratase (TylA2, 4 → 5),4 a 2-dehydrase (TylX3, 5 → 6),6 and a 3-reductase (TylC1, 6 → 7).6 Subsequently, 7 is transformed to 8 by methylation at C-3 by a methyltransferase (TylC3),7 8 is converted to 9 by epimerization at C-5 by an epimerase (TylK),4 and the reduction of 9 at C-4 by a reductase (TylC2)4 yields TDP-L-mycarose (3). Two constraints make a one-pot synthesis of 3 a necessity. First, compound 6 is inherently unstable since incubation of 5 with TylX3 results in its rapid consumption with the concomitant formation of TDP and maltol.7,8 Thus, the inclusion of TylC1 and NADPH in the reaction is required to reduce the labile 6 in situ to form the more stable product 7. Second, the in situ reduction catalyzed by TylC2 is necessary to drive the equilibrium of the epimerization products, 8 and 9, generated in the TylK reaction to completion, allowing the isolation of TDP-L-mycarose (3).4
Scheme 1.
To determine the feasibility of this plan, we purified TylX3,6 TylC1,6 TylC3,7 TylK, 4 and TylC24 to near homogeneity, along with RfbB, a TylA2 homologue in the rhamnose pathway from Salmonella typhi.9 The reaction was initiated by incubating 4 (7 mM) with RfbB (34 μM) in 50 mM potassium phosphate buffer (pH 7.5) at 37 °C for 1 h to generate 5.10 This conversion was nearly quantitative as indicated by HPLC analysis (Figure 1A, peak a).11 The remaining five mycarose biosynthetic enzymes were then added to the reaction mixture (30 μM each), together with NADPH (14 mM) and S-adenosyl-L-methionine (SAM, 7 mM). Incubation was continued at room temperature for 1 h. HPLC analysis11 and comparison of the isolated material (Figure 1B, peak b) with a standard (Figure 1C, peak b) showed that TDP-L-mycarose (3) was the major product. The overall yield of 3 from 4 was approximately 20%.
Figure 1.
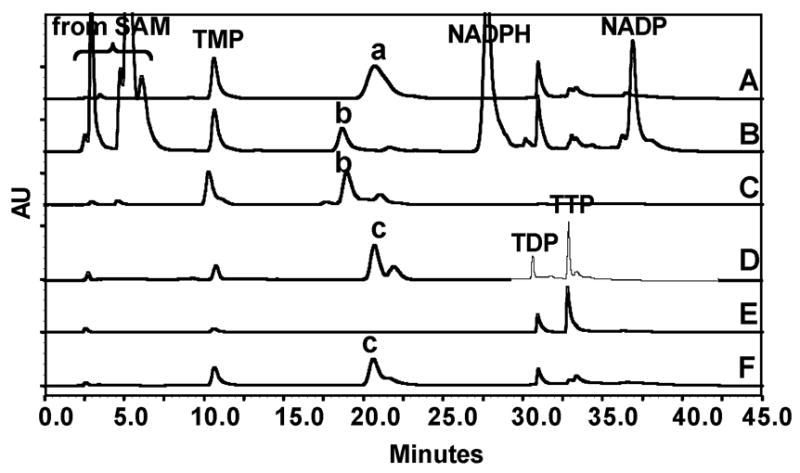
HPLC analysis of various incubation mixtures. (A) Incubation of TDP-D-glucose (4) with RfbB to generate 5 (peak a); (B) incubation of the reaction mixture from (A) and TylX3, TylC1, TylC3, TylK, TylC2, in the presence of NADPH and SAM to give TDP-L-mycarose (3) (peak b); (C) TDP-L-mycarose standard (peak c)4; (D) incubation of glucose-1-phosphate (10) and thymidine (12), PEP, ATP, MgCl2 with TK, TMK, NDK, PK, and RfbA to produce 4 (peak c); (E) incubation of thymidine (12), PEP, ATP, MgCl2 with TK, TMK, NDK and PK to make TTP (11); (F) incubation of the filtered reaction mixture from (E) with 10 and RfbA to give 4 (peak c).
The initial success in obtaining 3 using this method was encouraging. However, the high cost of TDP-D-glucose (4) limits its practicality. Hence, we explored the enzymatic generation of 4 for the biosynthesis of 3 in a one-pot preparation. As depicted in Scheme 1, TDP-D-glucose is produced via thymidylylation of 10 by glucose-1-phosphate thymidylyltransferase (TylA1 in the mycarose pathway of S. fradiae, or RfbA9 in the rhamnose pathway of S. typhi) using thymidine 5′-triphosphate (TTP, 11) as a co-substrate. In order to further reduce the cost, TTP was generated from thymidine (12) by the action of thymidine kinase (TK), thymidylate kinase (TMK), and nucleoside diphosphate kinase (NDK), as illustrated in Scheme 1.12 An ATP regenerating system mediated by pyruvate kinase (PK) was also included to facilitate the overall conversion.13
To assess the feasibility of making 4 enzymatically in one pot, a test reaction (12 mL) was carried out in which TK, TMK, NDK, PK, and RfbA (25 μM each) were incubated with thymidine (12, 20 mM), 10 (80 mM), phosphoenol pyruvate (PEP, 66 mM), ATP (2 mM), and MgCl2 (33 mM) in 50 mM Tris·HCl buffer (pH 7.5) at 37 °C for 6 h.14 However, the yield of 4 (Figure 1D, peak c)15 was low (46% calculated based on 12). This may have resulted from the hydrolysis of 4. To simplify the situation, the incubation reaction was conducted in two stages in which TTP was generated in the first stage and converted to TDP-D-glucose in the second stage. The first stage enzymes, TK, TMK, NDK, and PK, were removed by ultrafiltration (YM-10 membrane) after thymidine had been completely consumed (Figure 1E). The filtrate containing TTP was then incubated with 10 and RfbA at 37 °C for 6 h. The yield of 4 improved substantially (up to 85%) using this approach (Figure 1F, peak c).
Having solved the TDP-glucose production problem, we proceeded to synthesize 3 using thymidine and 10 as the starting materials. The initial reaction mixture contained four enzymes, TK, TMK, NDK, and PK (75 μM each), 3 mM 12, 12 mM PEP, 0.5 mM ATP and 10 mM MgCl2 in 100 μL of 50 mM Tris·HCl buffer (pH 7.5). After incubation at 37 °C for 10 min, the enzymes were removed by ultrafiltration (YM-10), and the filtrate was incubated with 3 mM 10 and 57 μM RfbA at 30 °C for 30 min. This mixture was subsequently treated with 28 μM RfbB, and the incubation was continued for 1 h at 37 °C. The mycarose biosynthetic enzymes, TylX3, TylC1, TylC3, TylK, and TylC2 (30 μM each), together with 6 mM NADPH and 3 mM SAM, were then introduced into the above reaction mixture. The final incubation was carried out at room temperature for 1 h. The yield of 16% for 3 was estimated based on HPLC analysis. This product was purified by FPLC on a MonoQ column (0–10 min, H2O; 10–20 min, a linear gradient from 0 to 280 mM ammonium bicarbonate buffer, pH 7.0) and desalted using a Sephadex G-10 column (2.5 × 50 cm, eluted with H2O at 24 mL/h). The identity of 3 was confirmed by 1H NMR and high resoluton MS analysis.4
In conclusion, this report describes a procedure combining six enzymes native to E. coli or S. typhi with five enzymes from S. fradiae that resulted in the biosynthesis of TDP-L-mycarose from the readily available precursors, glucose-1-phosphate and thymidine. This work is significant because (1) it demonstrated that an unstable TDP-sugar derivative can be successfully prepared by enzymatic synthesis; (2) while a total of 11 enzymes were used, the preparation starting from thymidine and glucose-1-phosphate is essentially a one-pot operation; (3) a convenient enzymatic method for the preparation of TDP-D-glucose, the common precursor for the biosynthesis of many unusual sugars, was developed; and (4) the results provided further in vitro evidence confirming the TDP-L-mycarose biosynthetic pathway. In addition, no apparent incompatibility on the reaction conditions for enzymes used in this multienzyme-synthesis is noted, and no obvious cross-inhibition caused by substrates/products generated in the incubation is observed. With the identification of many enzymes for the biosynthesis of a variety of unusual sugars now complete, the groundwork has been laid for investigations into the enzymatic preparation of these unusual sugars, a prerequisite for the in vitro glycodiversification of secondary metabolites. The two-stage one-pot approach described here can be readily applied to the synthesis of other unusual sugars.
Supporting Information Available
The construction of the expression plasmid, the isolation of the expressed enzymes, and incubation conditions to make 3 are presented. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the National Institutes of Health Grants GM35906 and GM54346.
References
- 1.He X, Liu H-w. Annu Rev Biochem. 2002;71:701–754. doi: 10.1146/annurev.biochem.71.110601.135339. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ozaki SI, Roessner CA, Stolowich NJ, Atshaves BP, Hertle R, Muller G, Scott AI. J Am Chem Soc. 1993;115:7935–7938. [Google Scholar]; (b) Schoevaart R, van Rantwijk F, Sheldon RA. J Org Chem. 2000;65:6940–6943. doi: 10.1021/jo000492y. [DOI] [PubMed] [Google Scholar]; (c) Corbett K, Fordham-Skelton AP, Gatehouse JA, Davis BG. FEBS Lett. 2001;509:355–360. doi: 10.1016/s0014-5793(01)03154-4. [DOI] [PubMed] [Google Scholar]
- 3.(a) Chen H, Guo Z, Liu H-w. J Am Chem Soc. 1998;120:9951–9952. [Google Scholar]; (b) Xue Y, Zhao L, Liu H-w, Sherman DH. Proc Natl Acad Sci USA. 1998;95:12111–12116. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao Z, Lin H, Liu H-w. J Am Chem Soc. 2005;127:7692–7693. doi: 10.1021/ja042702k. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H, Liu Y-n, Chen H, Liu H-w. J Am Chem Soc. 2005;127:9340–9341. doi: 10.1021/ja051409x. [DOI] [PubMed] [Google Scholar]
- 5.(a) Toshima K, Yoshida T, Mukaiyama S, Tatsuta K. Carbohydr Res. 1991;222:173–188. [Google Scholar]; (b) Lear MJ, Hirama M. Tetrahedron Lett. 1999;40:4897–4900. [Google Scholar]
- 6.Chen H, Agnihotri G, Guo Z, Que NLS, Chen XH, Liu H-w. J Am Chem Soc. 1999;121:8124–8125. [Google Scholar]
- 7.Chen H, Zhao Z, Hallis TM, Guo Z, Liu H-w. Angew Chem Int Ed. 2001;40:607–610. [PubMed] [Google Scholar]
- 8.Draeger G, Park SH, Floss HG. J Am Chem Soc. 1999;121:2611–2612. [Google Scholar]
- 9.Romana LK, Santiago FS, Reeves PR. Biochem Biophy Res Commu. 1991;174:846–852. doi: 10.1016/0006-291x(91)91495-x. [DOI] [PubMed] [Google Scholar]
- 10.An efficient method for the production of TDP-4-keto-6-deoxy-D-glucose (5) from TMP and 10 has been reported ( Oh J, Lee SG, Kim BG, Sohng K, Liou K, Lee HC. Biotech Bioeng. 2003;84:452–458. doi: 10.1002/bit.10789.
- 11.The incubation mixture was loaded on a Dionex PA1 column (5 μm, 4.6 × 250 mm) after ultrafiltration using a YM-10 membrane to remove the proteins. A linear gradient from 200 to 350 mM ammonium acetate buffer over 30 min (flow rate 0.6 mL/min) was used to elute the reaction products (monitored at 267 nm).
- 12.See Supporting Information for details.
- 13.Hirschbein BL, Mazenod FP, Whitesides GM. J Org Chem. 1982;47:3765–3766. [Google Scholar]
- 14.The workup procedure and the HPLC conditions were the same as those described in ref 11.
- 15.The yield was estimated by dividing the integration of peak c by the summation of integration of all peaks containing thymidyl substituent (TMP, TDP, TTP and 4). TDP-D-glucose (4) could be isolated using a P2 column (2.5 × 100 cm, elution with H2O at 6 mL/h). Its chromatographic behavior and spectral characteristics are identical to those of a commercial standard.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The construction of the expression plasmid, the isolation of the expressed enzymes, and incubation conditions to make 3 are presented. This material is available free of charge via the Internet at http://pubs.acs.org.


