Abstract
The continuous generation of new neurons in the adult hippocampus exhibits remarkable plasticity. Decreased neurogenesis is thought to underlie depression-like behaviors, and increased neurogenesis is thought to occur following antidepressant drug treatment. Studies on different strains of mice, however, yielded contrasting results with regard to the link between behavioral modifications induced by antidepressant drugs or environmental enrichment and changes in adult hippocampal neurogenesis. Therefore, we conducted a comparative study on the inbred strains Balb/c and C57Bl/6 that differ substantially in emotionality, stress reactivity, and behavioral responses to chronic antidepressant drugs. Quantitative assessments of progenitor cell proliferation and immature neuronal differentiation in the dentate gyrus revealed that, despite significantly different basal proliferation rates between both strains, neither strain exhibited changes in adult neurogenesis after exposure to early life stress or adult chronic fluoxetine treatment. A stimulatory effect of fluoxetine on adult hippocampal neurogenesis was only detected when treatment was initiated during adolescence, and this effect was abolished in mice exposed to early life stress, a prominent risk factor for developing adult-onset depression-like behaviors. Thus, in both strains of mice, neither adult fluoxetine treatment nor adolescent fluoxetine treatment following early life stress exposure increased the proliferation and early differentiation of adult neural progenitor cells.
Keywords: inbred mouse strains, depression, early life stress, fluoxetine, adult progenitor cell proliferation, adult progenitor cell differentiation
INTRODUCTION
New neurons continue to be born in the adult brain. In mammals, this type of adult neurogenesis is mainly restricted to the olfactory bulb and the dentate gyrus of the hippocampus (Lledo et al., 2006). In the hippocampus, adult progenitor cells have structural and molecular phenotypes of astrocytes that reside in the subgranular zone (SGZ) lining the hilar side of the granular cell layer (GCL; for review see Alvarez-Buylla and Lim, 2004). These progenitors give rise to functional neurons that project axons to the CA3 subfield and dendrites into the molecular layer (for reviews see Alvarez-Buylla and Lim, 2004; Ming and Song, 2005; Abrous et al., 2005; Overstreet-Wadiche and Westbrook, 2006).
In macaque monkeys, adult hippocampal neurogenesis generates about 0.004% of the total population of cells in the GCL per day. This is an order of magnitude lower than corresponding estimates in rodents at postnatal ages P60 to P90 (Kornack and Rakic, 1999). In the mouse, hippocampal neurogenesis is substantially higher in adolescents (P30) than in adults at P120, and adult hippocampal neurogenesis declines by an order of magnitude between 2 and 12 months of age (Bondolfi et al., 2004; He and Crews, 2007). Similar results were obtained from studies on rats, but it was noted that the age-related decline in adult neurogenesis did not result in a reduction of the total number of granule cells in the dentate gyrus (Lemaire et al., 2000). Nevertheless, adult neurogenesis has been extensively studied in mice and rats, and several studies have led to the prevailing view that adult hippocampal neurogenesis plays a role in human depression and its alleviation by antidepressant drugs. Influential examples include studies showing that chronic antidepressant treatment of rats and mice leads to increased proliferation and increased number of hippocampal neurons (Malberg et al., 2000; Wang et al., 2008). Moreover, in mice, behavioral effects of chronic antidepressant treatment appear to require adult neurogenesis (Santarelli et al., 2003). The potential relevance of these findings for depression is underscored by evidence showing that a prominent risk factor for developing depression, namely prenatal, early life, and adult stress, reduces hippocampal neurogenesis (Gould et al., 1998; Lemaire et al., 2000; Mirescu et al., 2004; Thomas et al., 2007), that subjects with recurrent major depression have decreased hippocampal volumes (Sheline et al., 1996; MacQueen et al., 2003), and that stress-induced changes in hippocampal volume and cell proliferation can be prevented by antidepressant treatment (Czéh et al., 2001).
A number of recent observations, however, are inconsistent with a functional role of adult hippocampal neurogenesis in depression and antidepressant drug action. Notably, despite of clear evidence for a survival-promoting effect of enrichment on newly born hippocampal neurons (Kempermann et al., 1997a), reduced anxiety- and depression-like behaviors induced by environmental enrichment were not dependent upon adult neurogenesis (Meshi et al., 2006). Moreover, not all mouse strains depend upon adult hippocampal neurogenesis to exhibit behavioral responses to antidepressant treatment (Holick et al., 2008; Huang et al., 2008). Thus, valid animal models of depression are needed to investigate further the significance of adult neurogenesis for changes in mood-related behavior and antidepressant drug actions. Such models should recapitulate salient characteristics of human depression such as a genetic predisposition that confers susceptibility for developing depression, increased stress reactivity (including persistent behavioral, endocrine and molecular alterations induced by adverse early life events), lower forebrain serotonin levels, and behavioral responsiveness to chronic (but not acute) antidepressant treatment (Manji et al., 2001; Nestler et al., 2002; Belmaker and Agam, 2008). From the different inbred strains of mice, Balb/c mice fulfill these requirements most closely (Dulawa et al., 2004; Zhang et al., 2004; Jacobson and Cryan, 2007; Huang et al., 2008). Moreover, Balb/c mice exposed to early life stress exhibit heightened depression-like behavioral responses in the adult forced swim test as well as gene expression changes in the adult forebrain (Bhansali et al., 2007) that were also detected in postmortem brains of subjects with major depression (Gurevich et al., 2002). However, in contrast to 129/Sv mice (Santarelli et al., 2003), Balb/c mice exhibit robust behavioral responses to chronic fluoxetine treatment in the absence of adult neurogenesis (Holick et al., 2008; Huang et al., 2008).
Because the interaction between early life stress and a genetic background conferring susceptibility for developing adult-onset depression has been implicated in the neurobiology of depression (Caspi et al., 2003), and because early life stress has been shown to decrease adult hippocampal neurogenesis in rats (Mirescu et al., 2004), we examined the proliferation and early differentiation of newly born hippocampal cells in Balb/c mice exposed to early life stress and raised to adulthood with and without antidepressant treatment and/or environmental enrichment, and compared these results to corresponding measures obtained from the stress-resistant C57Bl/6 mice.
MATERIALS AND METHODS
Animals
All experiments involving animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees at Columbia University and the New York State Psychiatric Institute. Male and female Balb/cJ and C57Bl/6J mice were housed in a facility with a 12-hour light/dark schedule (lights on at 6:00 am) with free access to food and water.
Infant Maternal Separation (IMS)
Offspring of first-time mothers were subjected to an IMS paradigm (Plotzy and Meaney, 1993) as previously described (Bhansali et al., 2007). Briefly, pups were separated from their dam daily for three hours (from 1:00 to 4:00 pm), starting at postnatal age day 2 (P2) and terminating at P15. Control animals were standard-facility-reared (SFR) offspring of first-time mothers. After weaning at P28, animals randomly selected from different litters were group-housed by sex. IMS mice were either treated with fluoxetine for 24 days during adolescence (from P32 to P56) or raised in an enriched environment during this time. The enrichment included post-weaning cross-housing Balb/c mice with the stress-resistant C57Bl/6 mice which has been shown to significantly reduce the heightened behavioral responses of adult IMS Balb/c mice to adult stress (Bhansali et al., 2007). Cages were also supplemented with igloos and cotton swabs to stimulate nest-building activities.
Chronic fluoxetine treatment
Mice received the drug with the drinking water for 24 days as described previously (Gurevich et al., 2002; Dulawa et al., 2004; Bhansali et al., 2007). During the last week of treatment with drinking water containing 10 mg/ml of fluoxetine, mice consumed an average of 16 mg/kg/day, a dose shown to lead to serum levels of fluoxetine that are equivalent to therapeutic doses used in humans (Dulawa et al., 2004). In additional experiments, mice were also treated with 10 or 25 mg/kg/day of fluoxetine. The consumption of drinking water containing these lower and higher concentrations of fluoxetine was equivalent to the consumption of drinking water containing 10 mg/ml of fluoxetine.
5-bromo-2′-deoxyuridine (BrdU) labeling
On days 22 and 23 of fluoxetine treatment or enrichment, animals and their respective controls were injected intraperitoneally (ip) with BrdU (Sigma-Aldrich, St Louis, MO) to label newly born cells. BrdU (75 mg/kg) was administered twice a day (at 2h interval) for two days (see Fig. 1).
Fig. 1.
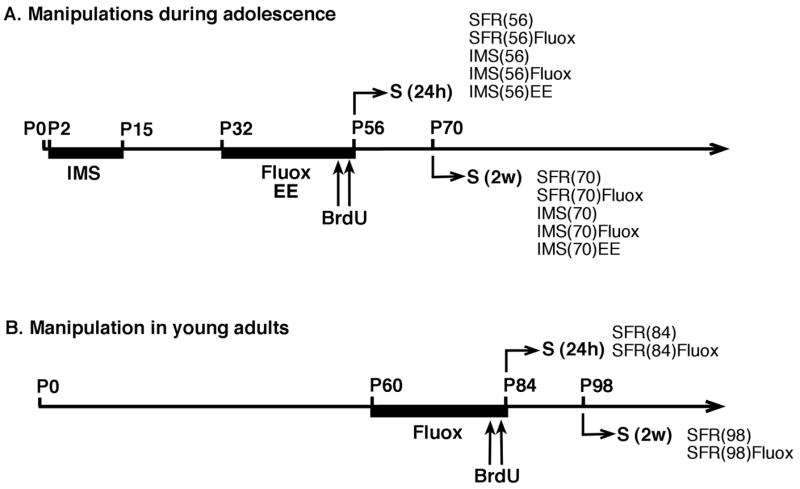
Timeline of experimental procedures. A. Mice subjected to IMS and their SFR controls received either fluoxetine (Fluox) or regular drinking water for 24 days during adolescence (P32 to P56) and were killed by transcardial perfusion (S) either 24 hours (24 h) or two weeks (2 w) after the last BrdU injection (75 mg/kg ip twice a day at 2 hours intervals on P54 and P55). Additional IMS-Balb/c mice were raised in an enriched postweaning environment (EE; from P32 to P56). B. SFR Balb/c and C57Bl/6 mice received fluoxetine or regular drinking water for 24 days during adulthood (P60 to P84) and were killed either 24 hours or 2 weeks after the last BrdU injection.
Tissue preparation for immuncytochemistry
Twenty four hours or two weeks after the last BrdU injection, mice were anesthetized with ketamine (100 mg/kg) and xylazine (15 mg/kg) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were removed and cryoprotected. For each brain, serial sections were collected to generate five representative sets of the entire hippocampus (−1.1 mm to −4.1 mm from bregma; Hof et al., 2000). Each set contained at least twenty 30-μm thick sections (150 μm apart) covering the entire anteroposterior extent of the hippocampus. Sections were processed either for BrdU or for doublecortin (DCX) immunostaining. Adjacent sections were counterstained with thionin to clarify the cytoarchitecture.
Quantification of BrdU-labeled cells
Free-floating sections were incubated for two hours in 50% formamide/2X sodium chloride/sodium citrate (SSC) at 65°C. After washing in 2X SSC, sections were incubated for thirty minutes in 0.3% H2O2 at room temperature to eliminate endogenous peroxidase. Sections were then washed in 0.1 M Tris-buffered saline (TBS, pH 7.5) and treated for thirty minutes in 2 N HCl at 37°C. Following this DNA denaturation step, sections were neutralized with borate buffer (0.1 M, pH 8.5), washed in TBS and blocked for two hours with 5% goat serum in TBS containing 0.5% Tween 20 (TBST) prior to an overnight incubation at 4°C with the mouse monoclonal anti-BrdU antibody diluted in TBST (1:1000; Becton-Dickinson, BD Biosciences, Pharmingen, San Jose, CA; clone 3D4; catalogue number: 555627; lot number: 52817). This antibody was raised against the immunogen BrdU, has been reported to be highly specific for BrdU, and does not cross-react with thymidine (Gratzner, 1982). In our control experiments, this antibody did not label tissues of non-BrdU-injected animals indicating that there was also no non-specific immunoglobulin binding.
After incubation with primary antibody, sections were washed in TBS and incubated for two hours with secondary antibody (biotin-conjugated goat anti-mouse IgG (H+L); 1:500; Jackson Immunoresearch Laboratories Inc., West Groves, PA; catalogue number: 115-065-003; lot number: 70355) diluted in TBS containing 5% goat serum. After washing in TBS, sections were incubated in avidin-biotin-peroxidase complex for one hour (ABC) (Vectastain Elite kit, 1:100 in PB; Vector Laboratories), followed by incubation for 7 minutes in 0.022% 3,3′-diaminobenzidine (DAB; Sigma) and 0.003% hydrogen peroxide in PB. All sections were rinsed in PB, mounted on gelatin-coated slides, air-dried and coverslipped.
For each mouse, all BrdU-labeled (BrdU+) cells detected in sections of the dentate gyrus of both hemispheres were counted at 40X magnification using a Zeiss Axioskop 2 microscope (Oberkochen, Germany) and, for each mouse, all 20 sections of one series (each 150 μm apart) were analyzed. Then, the mean count obtained was multiplied by 5 (the number of series) to obtain an estimate of the total number of BrdU+ cells throughout the entire dentate gyrus.
Quantification of DCX-immunolabeled cells
The number of DCX-expressing immature neurons was determined two weeks after the last BrdU injection. A guinea pig polyclonal anti-DCX antibody raised against a synthetic peptide comprising amino acids 350 to 365 of mouse DCX (Chemicon International, Temecula, CA; catalogue number: AB5910; lot number: 0512018147) was used in these experiments. On Western blots, this antibody recognizes a single protein band of 40 kDa which corresponds to the calculated molecular mass of DCX (Lee et al., 2003). Staining obtained with this antibody is identical to that described for other anti-DCX antibodies (Brown et al., 2003). DCX labeling was prominent in the dentate gyrus and DCX-labeled cells outside neurogenic regions of the brain were only rarely detected (see also Brown et al., 2003).
Free-floating sections were treated for thirty minutes with 0.3% H2O2 and blocked for two hours with 5% goat serum in TBST at room temperature. They were then incubated overnight at 4°C with the anti-DCX antibody (1:2,000 in TBST). On the following day, sections were washed in TBS and incubated for one hour with the secondary antibody diluted in TBS containing 5% goat serum (biotinylated goat anti-guinea pig IgG (H+L); 1:400; Vector Laboratories Inc., Burlingame, CA; catalogue number BA-7000; lot number: S0111). Detection of the secondary antibody was enhanced using the Vectastain ABC Elite kit, followed by applying DAB. Sections were rinsed in PB, mounted, air-dried and coverslipped.
To obtain estimates of the number of DCX-expressing cells, an unbiased stereological method, the optical fractionator, was used (West et al., 1991). For this analysis, a Zeiss Axioplan 2 photomicroscope (Oberkochen, Germany) equipped with an Optronics MicroFire Microscope Digital CCD camera (Goleta, CA) and Ludl motorized stage interfaced with a Dell computer and StereoInvestigator software (MBF Bioscience, Williston, VT) was used. For each mouse, DCX-labeled cells were counted in every 20th section (30 μm-thick, 600 μm apart) and a total of five sections, systematically representing the entire anteroposterior extent of the hippocampus, were analyzed. For each section, the contour of the GCL including the SGZ was first delineated at low magnification (10X). Counting was performed at high magnification in Koehler illumination conditions using a Zeiss 63X Plan-Apochromat 1.4 n.a. objective. DCX-labeled cells were systematically and randomly counted, using optical disector frames of 50×50 μm, counting grids of 100×100 μm, and a focusing range of 5.2 μm. These parameters yielded a coefficient of error, calculated as described previously (Schmitz and Hof, 2000), of less than 0.1. The volume of the dentate gyrus was estimated using the Cavalieri principle. All estimations were calculated for the left hippocampus and were doubled to represent the total hippocampal values.
For the production of photomicrographs, sections were photographed in Koehler illumination conditions. Images were first processed with Neurolucida software to systematically reduce background labeling and then processed using Adobe Photoshop (version 7.0) software for occasional adjustments of brightness and contrast.
Statistical analysis
Data were compared by one-way analysis of variance (ANOVA) and significant differences were resolved post hoc using Tukey Kramer Multiple Comparisons test.
RESULTS
Basal adult neurogenesis in Balb/c and C57Bl/6 mice
To compare basal rates of neurogenesis in adult Balb/c and C57Bl/6 mice, BrdU was injected twice daily for 2 days (at postnatal ages P54 and P55; see Materials and Methods) and the number of BrdU-labeled cells detected 24 hours post (last) BrdU injection was taken as a measure of progenitor cell proliferation. In addition, quantitative estimates of the number of BrdU- and doublecortin (DCX)-labeled cells, obtained two weeks after the last BrdU injection (i.e., in P70 animals), served as a measure of early survival and immature neuronal differentiation (see Fig. 1).
In both strains of mice, BrdU-labeled cells detected 24 hours post BrdU injection (at P56) were clustered in groups of two or four and, throughout the rostrocaudal extent of the dentate gyrus (DG), they were predominantly detected in the SGZ (Fig. 2). Two weeks after BrdU injection (at P70), BrdU-labeled cells were located mostly within the GCL and had large round nuclei (Fig. 3). A comparison between standard facility reared (SFR) Balb/c and C57Bl/6 mice revealed a significant difference in basal cell proliferation and differentiation. In Balb/c mice, the total number of BrdU-labeled cells in the dentate gyrus counted 24 hours post BrdU injection was 5,060 ± 111 (mean ± SD) and, in C57Bl/6 mice, this number was 10,092 ± 483 (two-tailed t test with Welch correction; t = 10.152, p < 0.001) (Fig. 2). Two weeks later (at P70), the number of BrdU-labeled cells declined about 1.7-fold in both strains, leading to an estimated total number of 2,955 ± 178 in Balb/c mice and 5,902 ± 267 in C57Bl/6 mice (t = 8.355, p < 0.001) (Fig. 3).
Fig. 2.
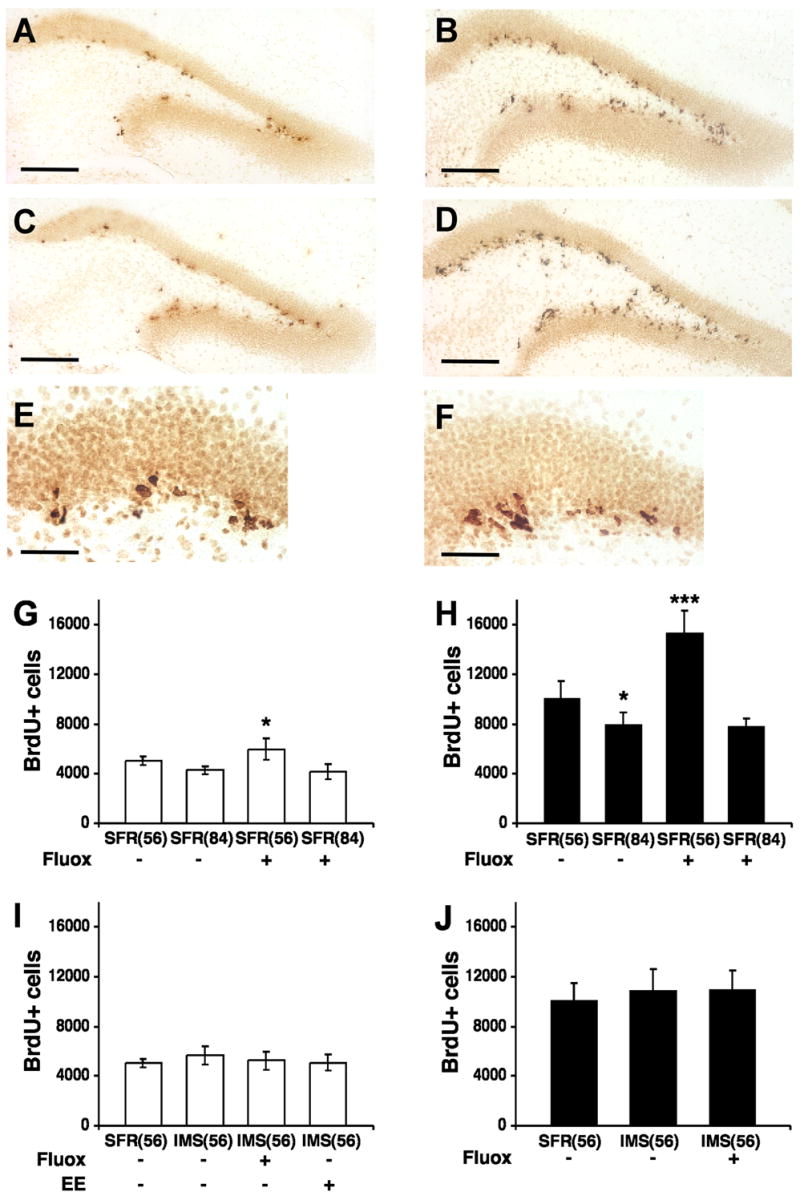
BrdU-labeled cells in the dentate gyrus of Balb/c and C57Bl/6 mice detected 24 hours after the last BrdU injection. A, Representative image of BrdU-labeled sections of the dentate gyrus of SFR Balb/c mice at postnatal age P56 that received regular drinking water. B, Representative image of BrdU-labeled sections of SFR C57Bl/6 mice (P56) that received regular drinking water. C, BrdU-labeling in SFR Balb/c mice (P56) after fluoxetine treatment during adolescence. D, BrdU-labeling in SFR C57Bl/6 mice (P56) after fluoxetine treatment during adolescence. E, BrdU-labeling in SFR Balb/c mice 24 hours post BrdU injection viewed at 40X magnification. F, BrdU-labeling in SFR C57Bl/6 mice 24 hours post BrdU injection (40X magnification). Scale bars: 200 μm (AD) and 50 μm (E, F). G, Number of BrdU-labeled (BrdU+) cells in SFR Balb/c mice at P56 and P84 that received either regular drinking water or fluoxetine (Fluox; 16 mg/kg/day) for 24 days during adolescence or adulthood. H, Number of BrdU+ cells in SFR C57Bl/6 mice at P56 and P84 that received either regular drinking water or Fluox for 24 days during adolescence or adulthood. I, Number of BrdU+ cells in SFR Balb/c mice and IMS Balb/c mice (P56) that received either regular drinking water or Fluox during adolescence, or were raised in an enriched environment (EE). J, Number of BrdU+ cells in SFR and IMS C57Bl/6 mice (P56) that received either regular drinking water or Fluox during adolescence. Data are means ± SD (n = 8–10 for non-treated animals and n = 5–6 animals for all other groups) of the total number of BrdU+ cells in the entire dentate gyrus and were compared by ANOVA followed post hoc by Tukey Kramer Multiple Comparisons tests. *p < 0.05; ***p < 0.001 compared with SFR controls.
Fig. 3.
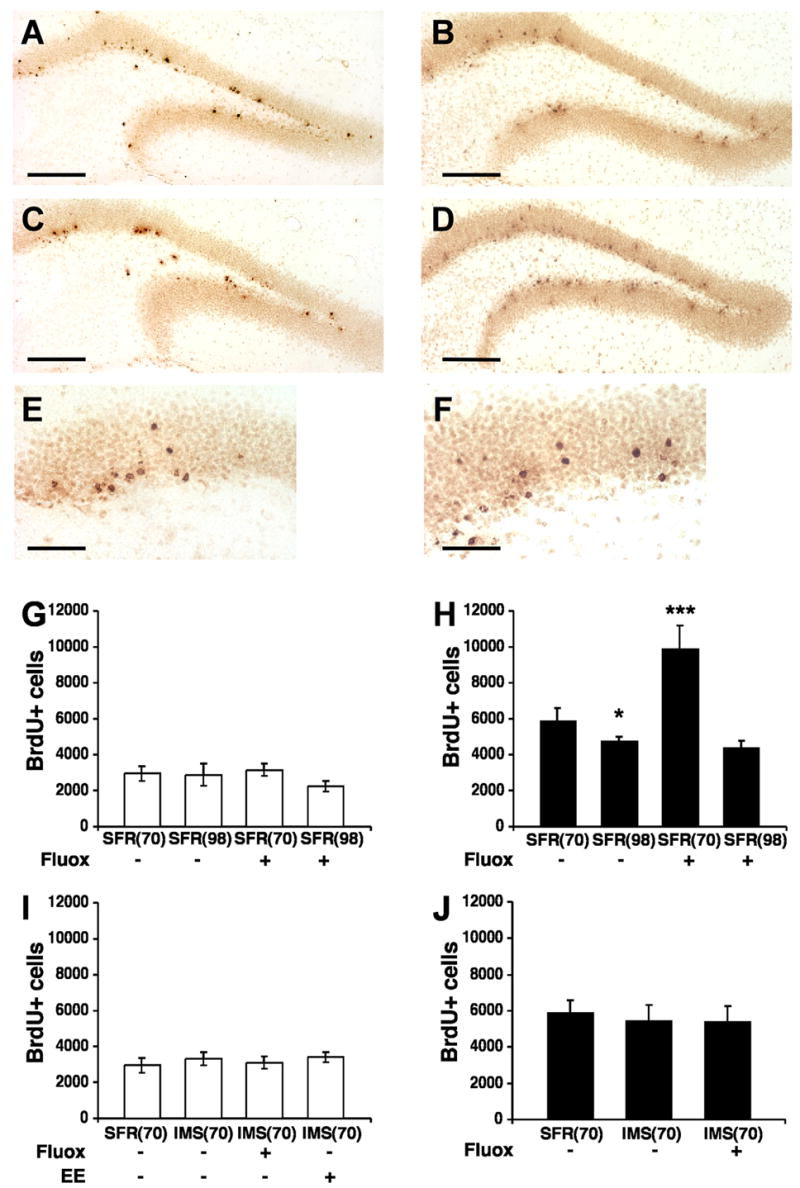
BrdU-labeled cells in the dentate gyrus of Balb/c and C57Bl/6 mice detected two weeks after the last BrdU injection. A, Representative image of BrdU-labeled sections of the dentate gyrus of SFR Balb/c mice at postnatal age P70 that received regular drinking water. B, Representative image of BrdU-labeled sections of SFR C57Bl/6 mice (P70) that received regular drinking water. C, BrdU-labeling in SFR Balb/c mice (P70) after fluoxetine treatment during adolescence. D, BrdU-labeling in SFR C57Bl/6 mice (P70) after fluoxetine treatment during adolescence. E, BrdU-labeling in SFR Balb/c mice 2 weeks post BrdU injection viewed at 40X magnification. F, BrdU-labeling in SFR C57Bl/6 mice 2 weeks post BrdU injection (40X magnification). Scale bars: 200 μm (AD) and 50 μm (E, F). G, Number of BrdU-labeled (BrdU+) cells in SFR Balb/c mice at P70 and P98 that received either regular drinking water or fluoxetine (Fluox, 16 mg/kg/day) during adolescence or adulthood. H, Number of BrdU+ cells in SFR C57Bl/6 mice at P70 and P98 that received either regular drinking water or Fluox during adolescence or adulthood. I, Number of BrdU+ cells in SFR and IMS Balb/c mice (P70) that received either regular drinking water or Fluox during adolescence, or were raised in an enriched environment (EE). J, Number of BrdU+ cells in SFR and IMS C57Bl/6 mice (P70) that received either regular drinking water or Fluox during adolescence. Data are means ± SD (n = 5–6 per group) of the total number of BrdU+ cells in the entire dentate gyrus and were compared by ANOVA following post hoc Tukey Kramer Multiple Comparisons tests. *p < 0.05; ***p < 0.001 compared with SFR controls.
DCX-immunolabeled cells were localized in the SGZ and GCL throughout the anteroposterior extent of the dentate gyrus (Fig. 4). Most DCX-labeled neurons had their soma located in either the SGZ or the inner third of the GCL and their dendrites extended into the outer two-thirds of the dentate molecular layer, thus revealing the phenotype of differentiated granule cells (Rao and Shetty, 2004). In Balb/c mice, the number of DCX-labeled cells (determined with the optical fractionator) was estimated at 36,531 ± 955 cells in the entire dentate gyrus. In C57Bl/6 mice, this number was 1.8-fold higher (67,143 ± 1,935) (t = 12.481, p < 0.001). Thus, consistent with results of previous studies (Kempermann et al., 1997b), the basal rate of progenitor cell proliferation is about 2-fold higher in C57Bl/6 compared with Balb/c mice. However, the basal neurogenesis rate (differentiation/proliferation ratio) is similar (0.58) in both strains, and so are the average volumes of their dentate gyri (Balb/c: 0.115 mm3, C57Bl/6: 0.117 mm3).
Fig. 4.
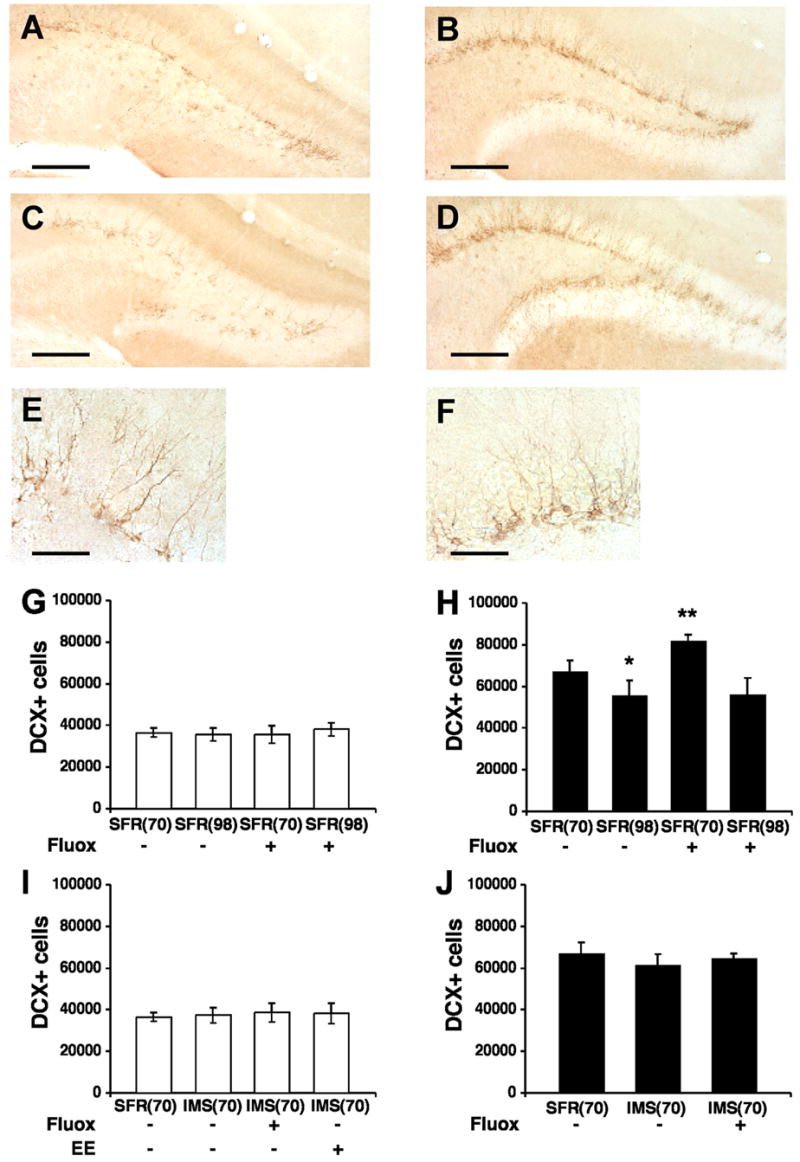
DCX-labeled cells in the dentate gyrus of Balb/c and C57Bl/6 mice detected two weeks after the last BrdU injection. A, Representative image of DCX-labeled sections of the dentate gyrus of SFR Balb/c mice at postnatal age P70 that received regular drinking water. B, Representative image of DCX-labeled sections of SFR C57Bl/6 mice (P70) that received regular drinking water. C, DCX-labeling in SFR Balb/c mice (P70) after fluoxetine treatment during adolescence. D, DCX-labeling in SFR C57Bl/6 mice (P70) after fluoxetine treatment during adolescence. E, DCX-labeling in SFR Balb/c mice 2 weeks post BrdU injection viewed at 40X magnification. F, BrdU labeling in SFR C57Bl/6 mice 2 weeks post BrdU injection (40X magnification). Scale bars: 200 μm (AD) and 50 μm (E, F). G, Number of DCX-labelled (DCX+) cells in SFR Balb/c mice at P70 and P98 that received either regular drinking water or fluoxetine (Fluox, 16 mg/kg/day) during adolescence or adulthood. H, Number of DCX+ cells in SFR C57Bl/6 mice at P70 and P98 that received either regular drinking water or Fluox during adolescence or adulthood. I, Comparison of the number of DCX+ cells in SFR and IMS Balb/c mice (P70) that received either regular drinking water or Fluox during adolescence, or were raised in an enriched environmen (EE). J, Number of DCX+ cells in SFR and IMS C57Bl/6 mice (P70) that received either regular drinking water or Fluox during adolescence. Data are means ± SD (n = 5–6 per group) of the total number of DCX+ cells in the entire dentate gyrus and were compared by ANOVA following post hoc using Tukey Kramer Multiple Comparisons tests. *p < 0.05; ***p < 0.001 compared with SFR controls.
In young adults of both strains, basal neurogenesis decreased significantly between P56 and P84 (one-way ANOVA, Balb/c: F(3,21) = 12.854; p < 0.001; C57Bl/6: F(3,19) = 39.076; p < 0.001). In C57Bl/6 mice, the number of newly born cells (determined 24 hours post BrdU injection) declined from 10,092 ± 483 at P56 to 7,952 ± 424 at P84 (post hoc Tukey Kramer Multiple Comparisons test: p < 0.05). In Balb/c mice, this number declined from 5,060 ± 111 at P56 to 4,280 ± 141 at P84 (p = 0.058) (Fig. 2). In C57Bl/6 mice, this age-related decline in neurogenesis was also evident when the numbers of BrdU-labeled cells (F(3,20) = 62.931, p < 0.001) and DCX-labeled cells (F(3,20) = 22.028, p < 0.001) were determined two weeks post BrdU injection (SFR(98): 4,756 ± 100 BrdU-labeled cells and 55,635 ± 3,551 DCX-labeled cells; p < 0.05 compared with SFR(70)). Corresponding measures in Balb/c mice, however, were not significantly different.
Effects of fluoxetine on adult neurogenesis following chronic adolescent and adult treatment
Adolescent (P32 to P56) and adult (P60 to P84) treatment with fluoxetine (see Fig. 1) had different effects on adult neurogenesis. In C57Bl/6 mice treated with 16 mg/kg/day of fluoxetine during adolescence, a robust and significant increase (F(3,19) = 39.076, p < 0.001) in the number of newly born cells was detected 24 hours post BrdU injection (15,372 ± 780) compared to SFR(56) controls (Fig. 2). Moreover, two weeks after BrdU injection, their numbers of BrdU-labeled cells (9,899 ± 578) (F(3,20)= 62.931; p < 0.001) and DCX-labeled cells (81,933 ± 1,252) (F(3,20) = 22.028; p < 0.001) were also significantly higher compared with corresponding controls (Figs 3 and 4). This stimulatory effect of adolescent fluoxetine on adult neurogenesis was further increased following treatment with 25 mg/kg of fluoxetine (F(2,12) = 121.49; p < 0.001). The numbers of newly born cells counted 24 hours and 2 weeks post BrdU injection were significantly higher (p < 0.001) compared with mice that received only 16 mg/kg of fluoxetine (Table 1).
TABLE 1.
Effect of adolescent fluoxetine treatment on the number of BrdU-labeled cells in SFR mice
| Balb/c | C57Bl/6 | |||
|---|---|---|---|---|
| 24 h post-BrdU | 2 weeks post-BrdU | 24 h post-BrdU | 2 weeks post-BrdU | |
| Vehicle | 5,060 ± 352 | 2,955 ± 397 | 10,092 ± 1,366 | 5,902 ± 707 |
| Adolescent Fluox (16)1 | 5,951 ± 840 | 3,155 ± 354 | 15,372 ± 1,744 | 9,899 ± 1,292 |
| Adolescent Fluox (25)1 | 6,331 ± 394 | 2,714 ± 141 | 28,600 ± 1,499*** | 17,915 ± 3,203*** |
Dose of fluoxetine (Fluox) in mg/kg/day. Data are mean ± SD and were compared by one-way ANOVA. Statistical differences (16 versus 25 mg/kg of Fluox) were resolved post hoc using Tukey Kramer Multiple Comparisons tests.
p < 0.001 compared with adolescent Fluox (16).
In Balb/c mice, adolescent fluoxetine treatment (16 mg/kg) also increased the number of newly born cells (5,951 ± 375 BrdU-labeled cells detected 24 hours post BrdU injection) compared to SFR(56) controls (F(3,21) = 12.854; p < 0.001) (Fig. 2), but their numbers of BrdU- and DCX-labeled cells determined 2 weeks after BrdU injection (at P70) were not significantly altered compared with SFR(70) controls (BrdU: 3,155 ± 158; DCX: 35,507 ± 1,878) (Figs 3 and 4). Treatment with 25 mg/kg of fluoxetine also yielded a significant increase of the number of newly born cells counted 24 hours post BrdU injection (ANOVA, F(2,17) = 11.3, p < 0.001). This difference was only significant when compared with vehicle-treated controls (p < 0.01) but not when compared with mice that received 16 mg/kg of fluoxetine. Moreover, the numbers of BrdU-labeled cells counted 2 weeks post BrdU injection did not differ between mice that received vehicle, or 16 and 25 mg/kg of fluoxetine (F(2,10) = 0.41, p = 0.67) (Table 1).
In contrast to the stimulatory effects of adolescent fluoxetine treatment on neurogenesis of both strains, adult fluoxetine treatment at 16 mg/kg/day altered neither the rate of progenitor cell proliferation (Fig. 2) nor the numbers of surviving and differentiating cells (Figs. 3 and 4). A similar result was obtained when the number of BrdU-labeled cells was estimated in mice that received either 10 or 25 mg/kg of fluoxetine. In both strains, neither dose led to significantly increased numbers of BrdU-labeled cells at 24 hours or 2 weeks post BrdU injection. Although 25 mg/kg of fluoxetine administered to C57Bl/6 mice led to increased numbers of newly born cells counted 24 hours post BrdU injection, this increase did not reach significance (Table 2).
TABLE 2.
Effect of adult fluoxetine treatment on the number of BrdU-labeled cells in SFR mice
| Balb/c | C57Bl/6 | |||
|---|---|---|---|---|
| 24 h post-BrdU | 2 weeks post-BrdU | 24 h post-BrdU | 2 weeks post-BrdU | |
| Vehicle | 4,280 ± 316 | 2,874 ± 604 | 7,952 ± 949 | 4,756 ± 246 |
| Adult Fluox (10)1 | 4,466 ± 222 | 2,504 ± 75 | 7,596 ± 215 | 4,017 ± 379 |
| Adult Fluox (16)1 | 4,143 ± 596 | 2,246 ± 298 | 7,846 ± 595 | 4,399 ± 370 |
| Adult Fluox (25)1 | 4,136 ± 190 | 2,238 ± 176 | 10,998 ± 3,483 | 5,955 ± 2,203 |
Dose of fluoxetine (Fluox) in mg/kg/day. Data are mean ± SD and were compared by one-way ANOVA. Statistical differences were resolved post hoc using Tukey Kramer Multiple Comparisons tests. No statistically significant differences were observed.
Adult hippocampal neurogenesis in mice exposed to early life stress
Offspring of Balb/c and C57Bl/6 mothers were subjected to infant maternal separation (IMS), a powerful paradigm of early life stress in rodents that elicits adult depression-like behaviors (Plotsky and Meaney, 1993; see Fig. 1). In our study, adult mice (P60) exposed to IMS had significantly lower (two-tailed t test, Balb/c: t = 3.485, p < 0.01; C57Bl/6: t = 7.492, p < 0.001) body weights compared with their standard-facility-reared (SFR) controls (SFR-Balb/c: 19.27 ± 0.77 g, IMS-Balb/c: 15.44 ± 0.67 g; SFR-C57Bl/6: 25.72 ± 0.45 g, IMS-C57Bl/6: 19.52 ± 0.76 g).
There was no significant difference in the number of BrdU-labeled cells counted 24 hours post BrdU injection (IMS-Balb/c: 5,663 ± 338, F(3,22) = 1.3; p = 0.3; IMS-C57Bl/6: 10,934 ± 765, F(2,16) = 0.7375; p = 0.5) (Fig. 2) and, two weeks later, the number of BrdU-labeled cells (IMS-Balb/c: 3,314 ± 163, F(3,17) = 1.9, p = 0.2 ; IMS-C57Bl/6: 5,445 ± 384, F(2,14) = 0.7, p = 0.5) and DCX-labeled cells (IMS-Balb/c: 37,439 ± 1,608, F(3,17) = 0.3, p = 0.8; IMS-C57Bl/6: 61,196 ± 2,528, F(2,14) = 2.4, p = 0.13) did also not significantly differ from their respective controls (Figs 3 and 4). Hence, early life stress exposure had no effect on progenitor cell proliferation and differentiation of newly born cells in the adult hippocampus.
Lack of stimulatory effects of adolescent fluoxetine on adult neurogenesis in IMS mice
In contrast to SFR mice, neither Balb/c nor C57Bl/6 mice subjected to IMS and treated with fluoxetine during adolescence exhibited altered cell proliferation and differentiation of newly born cells in the adult dentate gyrus. After treatment with 16 mg/kg of fluoxetine, the number of BrdU-labeled cells counted 24 hours post BrdU injection (Fig. 2) as well as the numbers of BrdU-labeled and DCX-labeled cells counted two weeks later (Figs 3 and 4) did not significantly differ from their respective controls. Moreover, 25 mg/kg of fluoxetine did also not alter the number BrdU-labeled cells counted 2 weeks post BrdU injection (Balb/c: F(2,11) = 0.83, p = 0.46; C57Bl/6: F(2,12) = 0.4, p = 0.68) (Table 3).
TABLE 3.
Effect of adolescent fluoxetine treatment on the number of BrdU-labeled cells in IMS mice
| Balb/c | C57Bl/6 | |||
|---|---|---|---|---|
| 24 h post-BrdU | 2 weeks post-BrdU | 24 h post-BrdU | 2 weeks post-BrdU | |
| Vehicle | 5,663 ± 757 | 3,314 ± 363 | 10,934 ± 1,710 | 5,445 ± 859 |
| Adolescent Fluox (16)1 | 5,231 ± 726 | 3,112 ± 321 | 10,946 ± 1,513 | 5,414 ± 868 |
| Adolescent Fluox (25)1 | nd | 3,301 ± 281 | nd | 4,773 ± 1,984 |
Dose of fluoxetine (Fluox) in mg/kg/day. Data are mean ± SD and were compared by one-way ANOVA. Statistical differences were resolved post hoc using Tukey Kramer Multiple Comparisons tests. No statistically significant differences were observed. nd: not determined.
No effect of environmental enrichment on adult neurogenesis in IMS Balb/c mice
We have previously shown that IMS Balb/c mice housed with SFR C57Bl/6 mice postweaning exhibit increased body weights and reduced responses to adult stress (Bhansali et al., 2007). Also in the present study, the body weights of IMS Balb/c mice exposed to postweaning enrichment (IMS-EE) were significantly higher (two-tailed t test, t = 2.995, p < 0.01) compared to non-enriched IMS mice (IMS 15.44 ± 0.67 g, IMS-EE: 18.85 ± 0.92). However, this enrichment altered neither the progenitor cell proliferation nor their early differentiation in the adult dentate gyrus. The number of newly born cells (determined 24 hours post BrdU injection; Fig. 2) and the numbers of BrdU- and DCX-labeled cells counted two weeks later (Figs 3 and 4) did also not differ significantly from their respective IMS controls (IMS(56) and IMS(70)).
DISCUSSION
Consistent with previous findings (Kempermann et al., 1997b), the proliferative activity of neuronal progenitor cells of C57Bl/6 mice was significantly (~2-fold) higher compared with Balb/c mice. Although the genetic background appears to determine the baseline rate of progenitor cell proliferation, both strains exhibited similar adult neurogenic responses to three different manipulations. Neither early life stress nor adult fluoxetine treatment altered the rate of progenitor cell proliferation and the number of immature neurons. Adolescent fluoxetine treatment, however, increased progenitor cells proliferation in both strains and, in C57Bl/6 mice, the number of differentiating neurons was also significantly increased. Hence, in Balb/c and C57Bl/6 mice, dentate gyrus progenitor cells differ in their capacity to respond to fluoxetine during maturation, and only adolescent fluoxetine stimulates progenitor cell proliferation. This age-dependent window for a stimulatory effect of fluoxetine on progenitor cell proliferation could be restricted to certain strains of mice since a stimulatory effect of adult fluoxetine treatment on progenitor cell proliferation has been reported for 129Sv mice (Santarelli et al., 2003). Nevertheless, in both strains studied here, the stimulatory effect of adolescent fluoxetine on progenitor cell proliferation is abolished when these mice were exposed to early life stress, a prominent risk factor for developing adult-onset depression-like behaviors.
Because depression is an adult-onset disorder with a mean age of onset in the mid-twenties, it is critical that the effects of antidepressant drugs on adult neurogenesis be assessed after adult treatment. Although in short-lived animals (rats and mice) the onset of sexual maturity (~P60) may preceed neuronal maturity, our data indicate that fluoxetine treatment of young “adults” already fails to stimulate hippocampal neurogenesis. This finding is unlikely to differ for older mice with precipitously lower basal neurogenesis rate (Bondolfi et al., 2004). Moreover, the lack of effect of adult fluoxetine treatment on progenitor cell proliferation was evident for three different doses of fluoxetine, two of which (10 and 16 mg/kg/day in drinking water) have been shown to yield plasma levels that are similar to those achieved with clinically effective doses of fluoxetine (Prozac), and the third dose (25 mg/kg/day) yields plasma levels that are ~3 times higher (Dulawa et al., 2004). Moreover, neither 24-hour nor 2-week post BrdU-injection measures indicate altered proliferation in mice treated with fluoxetine during adulthood (although a stimulatory effect on proliferation is clearly detected in adult SFR mice treated with fluoxetine during adolescence). Our measures of BrdU-labeled cells are also consistent with measures of DCX-labeled cells. One remote and untested possibility that could account for our findings is that the progenitor cells of C57Bl/6 and Balb/c mice develop tolerance or habituate faster to fluoxetine treatment. However, if this were the case, the previously proposed link between a stimulatory effect of fluoxetine on neural progenitor cell proliferation and antidepressant drug action (Malberg et al., 2000; Wang et al., 2008) would not hold up, especially since Balb/c mice have been shown to exhibit robust behavioral responses to fluoxetine (Dulawa et al., 2004).
An unexpected finding of the present study was the lack of an effect of early life stress on basal progenitor cell proliferation and early differentiation of newly born cells in the adult hippocampus. At first glance, this seems contrary to findings made in rats (Mirescu et al., 2004). However, in both strains of mice, early life stress abolished neurogenic responses to adolescent fluoxetine treatment. Thus, in both rats and mice, early life stress affects adult neurogenesis via different mechanisms. It decreases basal progenitor cell proliferation and survival rate in the rat and inhibits progenitor cell proliferation stimulated by adolescent fluoxetine in the mouse. Daily measures of drug-containing water consumption of IMS mice did not differ from SFR controls. Hence, the possibility that IMS mice drank less and thus, received less of the drug could be excluded. Another possibility, namely that drug absorption and/or the rate of metabolism differ between IMS and SFR mice is also not supported by our data showing that increasing the dose of fluoxetine to 25 mg/kg/day had also no effect on adult neurogenesis of IMS mice.
Together with previous findings, the present results indicate a clear divergence between adult hippocampal neurogenesis and depression-like behavioral phenotypes. For example, we have previously shown that, compared to SFR Balb/c mice, IMS Balb/c mice exhibited heightened passive behavioral responses to the adult forced swim test (FST; Bhansali et al., 2007), a test with face and predictive validity for studies on depression-like behaviors (Dulawa et al., 2004). However, we show here that adult hippocampal neurogenesis does not differ between SFR and IMS mice. Moreover, although previous studies revealed that adolescent fluoxetine treatment and postweaning enrichment significantly reduced passive behavioral responses of IMS mice in the FST (Bhansali et al., 2007), the present study shows that none of these treatments affected hippocampal neurogenesis in IMS mice. Finally, our previous study on SFR mice also showed that adult, but not adolescent, fluoxetine treatment decreased their passive behavioral responses in the FST (Bhansali et al., 2007). However, in the present study, increased proliferation and early differentiation of hippocampal neurons were only detected after adolescent fluoxetine treatment of SFR mice.
In summary, in the IMS paradigm employed here, a link between depression-like behaviors in adulthood, antidepressant drug action, and altered progenitor cell proliferation and early differentiation in the adult hippocampus could not be established. It is possible that different types of animal models of depression exert their effects via different mechanisms. It remains therefore to be investigated whether the present findings also extend to other animal models of depression. Moreover, because adult neurogenesis is not just an increase in the number of neurons but a continuous source of new neurons with qualitatively different functional properties (Song et al., 2005), it remains to be tested whether the integration of mature new neurons in the adult hippocampus is critical for certain antidepressant effects to occur. The role of adult hippocampal neurogenesis in the pathophysiology of depression, however, will ultimately have to be demonstrated for human subjects.
Acknowledgments
We thank Deirdre DeSteno and Michael Saxe for their help and advise during the early stages of these experiments, and Bridget Wicinski and Devorah Segal for their help with experiments involving the optical fractionator.
Grant support:
Grant sponsor: National Institutes of Health; Grant numbers: MH061906, MH062185, and MH078993. Grant sponsor: Fondation Recherche Médicale; Grant number SPE20051105125 (to S.N.).
Literature cited
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the α subunit of the heterotrimeric G protein Gq. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57Bl/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa S, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork A, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;43:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol Biochem Behav. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57Bl/6 and 129/SV mouse brains. Amsterdam: Elsevier; 2000. [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in Balb/c mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang G-J, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiat. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: A review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn H, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997a;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997b;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E-J, Kim I-B, Lee E, Kwon S-O, Chun M-H. Differential expression and cellular localization of doublecortin in the developing rat retina. Eur J Neurosci. 2003;17:1542–1548. doi: 10.1046/j.1460-9568.2003.02583.x. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P-M, Alonso M, Grupp MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Neurosci. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Ming G-L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gold E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch A, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult brain. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficiency of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstraub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Kempermann G, Wadiche LO, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: Synaptogenesis and functional integration. J Neuroci. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-W, David D, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu J-M, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]


