Abstract
Autophagy has been associated with both cell survival and cell death, but the role of autophagy in cell death has been controversial. In this issue, Berry and Baehrecke (2007) report that autophagy is involved in physiological cell death during Drosophila development and is controlled by similar mechanisms as those that control its function in cell survival.
Autophagy is a conserved catabolic process that degrades long-lived proteins, organelles, and bulk cytoplasm (reviewed by Baehrecke, 2002; Maiuri et al., 2007). Autophagy is induced under conditions of stress such as starvation, hypoxia, heat, and drug treatment. A morphological hallmark of autophagy is the presence of autophagosomes—cytoplasmic vesicles that have a double membrane and contain cytoplasmic cargo to be degraded. Autophagosomes fuse with lysosomes to form autolysosomes in which the cytoplasmic cargo is digested. Finally, lysosomal permeases release the digested material back into the cytosol for recycling. In this capacity, autophagy sustains cell viability under starvation conditions for days and weeks.
Paradoxically, autophagy has also been implicated in a type of programmed cell death (type II PCD) called autophagic cell death that is different from apoptosis (type I PCD) (Schweichel and Merker, 1973). Autophagic cell death is also defined by the presence of autophagosomes and autolysosomes in dying cells. However, in contrast to autophagy—which is very well characterized and requires more than 20 autophagy (atg) genes (Xie and Klionsky, 2007)—little is known about the mechanisms and genes that regulate autophagic cell death under physiological conditions. In this issue, Berry and Baehrecke (2007) close this gap in our knowledge. These authors show that autophagic cell death occurs under physiological conditions during development and is controlled by similar mechanisms as autophagy in cell survival.
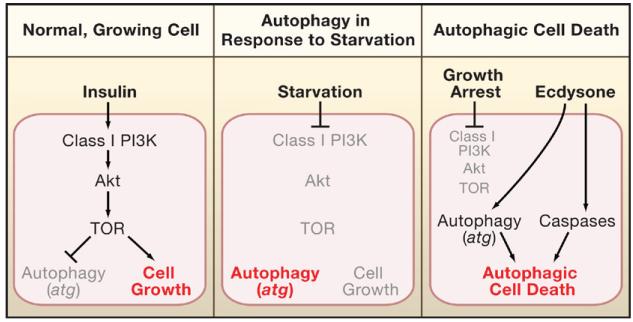
Key regulators of autophagy include the class I phosphoinositide 3-kinase (PI3K) (Blommaart et al., 1997). PI3-K regulates cell growth by sensing the availability of nutrients through growth factors such as insulin and is also regulated by the Ras pathway. Activated PI3K signals via Akt kinase to the TOR (target of rapamycin) kinase, which inhibits autophagy and promotes cell growth (Figure 1). Berry and Baehrecke tested whether these genes also regulate autophagic cell death. As an experimental model, they used salivary glands in Drosophila larvae, which secrete digestive enzymes during larval stages when the animal is heavily feeding. During pupal stages the animal stops feeding, and several larval structures including the salivary glands, the fat body, and the midgut are degraded and recycled to allow tissue remodeling and morphogenesis for the transformation of the worm-like larvae into the adult fly. The PCD of the salivary glands is triggered by the steroid hormone ecdysone and occurs extremely rapidly. The death of these glands exhibits the morphology of autophagic cell death. However, ecdysone also induces caspase activation in salivary glands, a hallmark of apoptosis (Figure 1), and it was unclear whether caspases contribute to autophagic cell death. Thus, salivary glands provide an excellent model to test the role of growth, autophagy (atg genes), and caspases for autophagic cell death under physiological conditions.
Figure 1. Cellular Responses to Different Environmental Conditions.
The cellular response is highlighted in red. Active factors are in black and inactive factors in gray.
(Left) In normal, growing cells, insulin promotes cell growth and inhibits autophagy through class I PI3K, Akt, and TOR signaling.
(Middle) In starving cells, PI3K, Akt, and TOR are not activated; thus, autophagy is derepressed.
(Right) Autophagic cell death of salivary glands is under control of the steroid hormone ecdysone, which induces expression of autophagic atg genes and apoptotic components such as caspases. Because salivary glands die during pupariation when the animals stop feeding, growth arrest occurs and blocks PI3K, Akt, and TOR activity.
Berry and Baehrecke first tested the role of growth and growth arrest in PCD in the salivary gland. Indeed, a marker of PI3K activity and hence growth is no longer detectable in salivary glands at the onset of pupariation, suggesting that growth arrest precedes salivary gland PCD. To establish a causal link between growth arrest and PCD in the salivary gland, p110 (the active subunit of PI3K), Akt and active Ras (RasV12) were expressed in salivary glands to inhibit growth arrest. Under these conditions, the salivary glands continued to grow and salivary gland tissue remained 24 hr after pupariation. Interestingly, the increase of salivary gland size is due to increase in cell volume, whereas the total number of cells remains constant. Thus, the authors conclude that growth arrest correlates with autophagic cell death. Expression of cell-cycle regulators such as Myc or CyclinD/Cdk4, which prevents apoptosis, did not prevent salivary gland PCD. Given that cell-cycle arrest correlates with apoptosis, this result further distinguishes autophagic cell death from apoptotic cell death.
Despite prolonged survival, salivary glands expressing p110, Akt, and RasV12 contain activated caspases and exhibit DNA fragmentation, both hallmarks of apoptosis. Thus, although maintaining growth conditions blocks autophagic cell death, the apoptotic component of autophagic cell death remains intact. Therefore, Berry and Baehrecke tested a requirement of caspases in autophagic cell death. Salivary glands are at least partially, if not completely, degraded in caspase mutants or in glands expressing the caspase inhibitor p35, suggesting that caspases are not strictly required for PCD in salivary glands. Nevertheless, combined expression of the active subunit of PI3K p110 and the caspase inhibitor p35 results in stronger persistence of salivary glands compared to expression of either alone. Thus, both growth arrest and caspases contribute to autophagic cell death of salivary glands. Furthermore, this observation also implies that additional caspase-independent factors are required for salivary gland PCD.
Good candidates for the caspase-independent factors are the atg genes, which are essential for starvation-induced autophagy and which are upregulated in dying salivary glands (Gorski et al., 2003). Berry and Baehrecke found that mutants or RNA interference of seven different atg genes display incomplete degradation of salivary glands 24 hr after pupariation, providing the first in vivo evidence that autophagy and atg genes are required for autophagic cell death. These data also indicate that autophagic cell death is not a failed survival attempt of dying cells, because if it were then the salivary glands would die in atg mutants. Overexpression of Atg1 induces premature onset of salivary gland destruction consistent with a previous report in the fly fat body (Scott et al., 2007). Thus, autophagy is both necessary and sufficient for autophagic cell death of salivary glands. Interestingly, salivary glands lacking atg also contain active caspases and the combined inhibition of both autophagy and caspases results in more intact salivary glands than inhibition of either alone. Thus, both autophagy and caspases contribute to autophagic cell death of salivary glands (Figure 1).
In conclusion, this study demonstrates that growth arrest, autophagy, and caspases contribute to physiologically occurring autophagic cell death. Several questions remain; for instance, how is the life-or-death decision of autophagy controlled? Good candidates for the decision makers are caspases. It is noteworthy that caspase activity has been observed in other models of autophagic cell death (mammary lumen formation, embryonic cavitation, and amphibian development). Thus, it is possible that caspase activity in autophagy may tip the balance from survival to death.
Another question is, why do salivary glands die by an autophagic process despite containing active caspases? Caspases do not always induce apoptosis and have important functions outside of apoptosis (Kuranaga and Miura, 2007). Participation in autophagic cell death may be another example of a nonapoptotic function of caspases. However, it is unknown how caspases trigger nonapoptotic responses.
It is now clear that autophagy participates in cell death under some circumstances and promotes cell survival in others. Although this presents a dichotomy, any resulting confusion is largely resolved if autophagy is simply viewed as a catabolic process that provides energy and resources to many cellular and biological processes. In some cases, this may promote cell survival; in others it may facilitate cell death. This context-dependent behavior of autophagy may also be relevant for human health. Autophagy sometimes prevents and sometimes promotes cancer (Levine, 2007). Because drug treatment can induce autophagy, it will be important to determine which types of cancer use autophagy for survival and which ones undergo autophagic cell death. Thus, the study by Berry and Baehrecke demonstrates the importance of a careful case-by-case evaluation of autophagy.
ACKNOWLEDGMENTS
I would like to thank the NIH (GM068016, GM074977, GM081543) and The Robert A. Welch Foundation (G1496) for support.
REFERENCES
- Baehrecke EH. Nat. Rev. Mol. Cell Biol. 2002;3:779–787. doi: 10.1038/nrm931. [DOI] [PubMed] [Google Scholar]
- Berry DL, Baehrecke EH. Cell. 2007 this issue. [Google Scholar]
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. Eur. J. Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Gorski SM, Chittaranjan S, Pleasance ED, Freeman JD, Anderson CL, Varhol RJ, Coughlin SM, Zuyderduyn SD, Jones SJ, Marra MA. Curr. Biol. 2003;13:358–363. doi: 10.1016/s0960-9822(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Miura M. Trends Cell Biol. 2007;17:135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Levine B. Nature. 2007;446:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Schweichel JU, Merker HJ. Teratology. 1973;7:253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- Scott RC, Juhasz G, Neufeld TP. Curr. Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Nat. Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]