Abstract
Certain patterns of anger expression have been associated with maladaptive alterations in cortisol secretion, immune functioning, and surgical recovery. We hypothesized that outward and inward anger expression and lack of anger control would be associated with delayed wound healing. A sample of 98 community-dwelling participants received standardized blister wounds on their non-dominant forearm. After blistering, the wounds were monitored daily for eight days to assess speed of repair. Logistic regression was used to distinguish fast and slow healers based on their anger expression pattern. Individuals exhibiting lower levels of anger control were more likely to be categorized as slow healers. The anger control variable predicted wound repair over and above differences in hostility, negative affectivity, social support, and health behaviors. Furthermore, participants with lower levels of anger control exhibited higher cortisol reactivity during the blistering procedure. This enhanced cortisol secretion was in turn related to longer time to heal. These findings suggest that the ability to regulate the expression of one’s anger has a clinically relevant impact on wound healing.
Keywords: Anger Expression, Anger Control, Wound Healing, Cortisol, Hostility, Negative Affect, Social Support, Health Behaviors, Psychoneuroimmunology
1. Introduction
1.1 Stress and Wound Healing
Both brief naturalistic stressors and chronic stress are associated with delayed wound healing. For example, women who were caring for a spouse or parent diagnosed with dementia took on average 24% longer to heal a standardized wound compared to demographically indistinguishable control participants (Kiecolt-Glaser et al. 1995). Milder transitory stressors can also influence wound repair. Dental students took 40% longer to heal a wound placed on the hard palate before an examination compared to a wound placed at the end of their summer vacation (Marucha et al. 1998).
Self-reported symptoms of psychological distress appear to affect speed of healing as well. Healthy young men who were classified as slow healers, i.e. who were in the half of the sample that took longer to heal, reported significantly more perceived stress than fast healers (Ebrecht et al. 2004). Similarly, patients with leg ulcers who exhibited higher levels of anxiety and depression were more likely to be categorized as slow healers, compared to patients reporting lower psychological distress (Cole-King and Harding, 2001).
Even a very commonplace stressor, a discussion of marital problems, can delay wound healing. Couples took one day longer to heal blister wounds placed following discussion of a marital disagreement compared to wounds placed before a supportive interaction. Importantly, the couples who exhibited high levels of hostile behaviors across both visits took two more days to heal on average compared to less hostile couples. Following the marital interaction, hostile couples also reported higher levels of negative mood for the remainder of the day compared to couples displaying fewer negative behaviors; this increased negative mood occurred despite an absence of affective group difference at baseline, suggesting a lingering negative impact of the marital disagreement (Kiecolt-Glaser et al. 2005).
1.2 Anger and Wound Healing
Anger is among the emotions elicited by conflicted interaction that might contribute to slower wound repair. This affective state includes feelings that range in intensity from irritation or annoyance to intense fury and rage (Spielberger et al. 1983). Trait anger represents the propensity to frequently experience feelings of anger (Spielberger et al. 1983), while hostility refers to a negative attitude toward others, consisting of enmity, denigration, and ill will (Smith, 1994). A hostile attitude appears to lead to more frequent episodes of anger (Eckhardt et al. 2004). The manner in which the anger feelings are expressed appears to influence the physiological and psychological consequences of this negative emotion (Deffenbacher et al. 1996). The tendency to express one’s anger outwardly toward individuals or objects through physically or verbally aggressive behaviors has been termed “anger out”, while “anger control” refers to the extent that an individual attempts to control the outward expression of anger. In contrast, “anger in” is defined as the tendency to suppress angry feelings (Spielberger, 1988). Anger expression styles have been conceptualized as stable traits (Eckhardt et al. 2004).
In naturalistic studies, higher self-reported anger and anger suppression have been associated with a longer postoperative recovery and more post-surgical complications (Sharma et al. 2007; Stengrevics et al. 1996). Those data suggest that anger might contribute to delayed wound healing. In addition, the expression of anger has been related to immune dysregulation. For example, family dementia caregivers who display high levels of anger out and low levels of anger control had a lower proliferative response to two mitogens than caregivers with low anger out and high anger control (Scalan et al. 2001). Conversely, cardiac patients who had better control over the expression of their anger had higher natural killer (NK) cell cytotoxicity than patients who had poorer anger control (Ishihara et al. 2003). Furthermore, among men with prostate carcinoma, anger suppression has been associated with decreased NK cell cytotoxicity (Penedo et al. 2006).
Differences in pattern of anger expression have also been associated with cortisol secretion, an important endocrine modulator of wound healing. Momentary assessment studies revealed that feelings of anger and their expression are associated with cortisol elevations 20 minutes later (Adam, 2006; Adam et al. 2006). In a similar vein, hostile men who directed their anger toward their spouse during a marital interaction task exhibited higher glucocorticoid secretion than men who did not express angry feelings (Miller et al. 1999). Teachers experiencing high levels of job strain who tend to express their anger outwardly displayed higher early morning cortisol elevations compared to teachers who reported low levels of anger out (Steptoe et al. 2000). In addition, healthy volunteers frequently reporting suppression of their anger secreted more cortisol following acute laboratory psychological stressors (Larson et al. 2001).
Data from both human and animal models have linked enhanced glucocorticoid secretion with slower wound healing. Higher elevations in morning cortisol were observed in healthy men who were categorized as slow healers compared to those who were considered fast healers (Ebrecht et al. 2004). Similarly, higher levels of salivary cortisol were associated with lower production at the wound site of two cytokines essential for the integrity of the repair process (Glaser et al. 1999). In animal models, a glucocorticoid receptor antagonist attenuated stress-related decrements in wound repair, confirming the role of this stress hormone in delayed wound healing (Padgett et al. 1998; Detillion et al. 2004).
Several studies suggest that the health consequences associated with different patterns of anger expression differ by gender. For example, outward expression of anger has been associated with altered glucose metabolism among women, but not men (Suarez, 2006). Similarly, a curvilinear relationship between anger in and systolic blood pressure has been described among women, while anger suppression had no impact on blood pressure among men (Hogan and Linden, 2005). In addition, sex differences in cortisol responses to stress have been reported. Some studies suggest that after puberty, but before menopause, women tend to exhibit less cortisol reactivity to laboratory psychological stressors than men (Kajantie and Phillips, 2006; Kudielka and Kirschbaum, 2005). Therefore, gender might moderate the impact of anger expression on wound healing.
1.3 Relaxation and Wound Healing
This study was part of a project examining the effects of relaxation on wound repair. Psychological preparation before surgery appears to promote better adjustment and recovery in clinical settings (Kiecolt-Glaser et al. 1998). Moreover, relaxation interventions seem to facilitate recovery following surgery (Montgomery al. 2002). However, the physiological mechanisms underlying the effect of relaxation are largely unknown. Experimental studies suggest that psychological stress is associated with dysregulation of cytokines production at the wound site (Glaser et al., 1999; Kiecolt-Glaser et al., 2005). Nevertheless, it remains unclear whether a relaxation intervention promotes faster healing by influencing inflammatory mediators of the wound repair process.
1.4 The Present Study
Certain patterns of anger expression have been associated with maladaptive alterations in cortisol, immune functioning, and surgical recovery. Accordingly, we hypothesized that the style of anger expression could distinguish between fast and slow healers. Specifically, we tested the hypothesis that individuals who tend to frequently express their anger outwardly or inwardly and those who have difficulty controlling the expression of their anger would be more likely to be categorized as slow healers, compared to participants with low anger out, low anger in, and high anger control. Given the known sex differences in the impact of anger expression on health outcomes, we also tested whether the impact of anger expression on wound healing was moderated by gender. We also hypothesized that higher levels of outward and inward anger expression and lack of control over the expression of anger would be associated with increased cortisol secretion and, in turn, more time to heal. Since production of proinflammatory cytokines appears to be modulated by cortisol (Glaser et al. 1999), we also examined the relation of IL-1α, IL-1β, IL-6, IL-8, and TNF-α at the wound site to the mode of anger expression.
This study was part of a research project examining the physiological mechanism by which relaxation can facilitate wound healing. It was hypothesized that individuals randomized to the relaxation intervention would heal faster compared to the control condition. In addition, we also hypothesized that the relaxation intervention would exert its action by influencing early inflammatory events at the wound site.
2. Methods
2.1 Participants
Participants were part of a larger study on the effects of relaxation on wound repair. Individuals were recruited from newspaper advertisements, area newsletters, church groups, university alumni publications, and word of mouth. Following an initial screening interview, individuals were excluded if they reported health problems or related medications that had an immunological or endocrinological component (eg, cancer, recent surgeries, strokes, diabetes mellitus, peripheral vascular disease, conditions such as asthma or arthritis that required regular use of anti-inflammatories). Applicants were also excluded if they smoked, used alcohol (more than 10 drinks per week for women or more than 20 drinks per week for men) or caffeine excessively (more than 10 cups per day), were more than 30% above or 10% below their ideal weight, reported engaging in any regular relaxation practice, or reported any prior allergic responses to bandages, tapes, or adhesives. Additional exclusion criteria included needle, hospital, or blood phobias, previous psychiatric hospitalization, use of psychotropic medication or severe psychological distress within the last three months. Although minority participants were not excluded from this study, they were not actively recruited because of the risk of long-term skin discoloration associated with the experimental procedure among non-Caucasian (Surber et al. 1999). This risk was clearly described, during the screening interview and in the informed consent. The Ohio State University Biomedical Research Review Committee approved the project; all subjects gave written informed consent prior to participation. Socio-demographic characteristics of the participants included in the study can be found in Table 1. The final sample included 10 women who were post-menopausal; two of them were taking hormone replacement therapy.
Table 1.
Socio-demographic characteristics of the participants
| Sex of the participants | |||
|---|---|---|---|
| Age | Male | Female | Total |
| < 30 | 18 | 16 | 34 |
| 30–49 | 15 | 28 | 43 |
| 50–64 | 5 | 12 | 17 |
| 65+ | 2 | 2 | 4 |
| Total | 40 (40.8%) | 58(59.2%) | 98 |
|
| |||
| Ethnicity | |||
|
| |||
| Caucasian | Non-Caucasian | ||
| 80 (81.6%) | 18 (18.4%) | ||
2.2 Study Design
The standardized wounding procedure was performed during a 26-hour admission at the OSU General Clinical Research Center (GCRC). Participants were randomized into two groups: a relaxation training and a control group. Following the GCRC admission, wounds were monitored daily for eight days to assess the speed of healing. Participants were paid up to $550 if they completed the GCRC admission and all the follow-up visits.
2.3 Overview, GCRC Admission
At 7:00 AM, following an overnight fasting, participants were admitted to the GCRC, fed a standard breakfast, and given questionnaires to complete. A heparin well was inserted in each participant’s arm, and a baseline blood sample was drawn to provide serum for the blister wells. Nurses attached the vacuum pump and template to raise blisters on the non-dominant arm, a process that took about one hour (see the Suction Blister Model section).
To assure consistent physical activity, participants remained in the room over the 26 hours. Other questionnaires were completed after lunch and following every relaxation session. Salivary cortisol samples were obtained every hour from 7:00 AM through 10:00 PM; additional samples were also collected during the attachment of the pump and the blister chamber. Samples were centrifuged and frozen at −70C until assayed.
The first relaxation session occurred shortly after the beginning of the blistering. Subsequent sessions were conducted at one, four, and eight hours after the blister chamber had been strapped to the subject’s arm. Relaxation strategies included progressive muscular relaxation, imagery, breathing techniques and self-hypnosis. Relaxation sessions lasted about 45 minutes and were conducted by an experienced clinical psychologist and well-trained graduate students. Control participants were asked to sit quietly during those times.
2.4 Self-Report Questionnaires
The Anger Expression Scale provided a way to categorize differences in anger expression (Spielberger, 1988). This 24-item scale yields three factor analytically-derived subscales. The anger out subscale evaluates the frequency that feelings of anger are expressed in aggressive behaviors directed toward other people or objects in the environment (eg. I lose my temper; I strike out at whatever infuriates me). The anger-control subscale assesses the frequency that the person monitors and prevents the outward expression of anger (eg. I control my temper; I keep my cool). The anger in subscale assesses the frequency that angry feelings are held in or suppressed (e.g. I keep things in; I boil inside, but I don’t show it). Participants rated the frequency to which they would respond in a specific manner when they were angry or furious on a four-point likert scale (1- almost never to 4- almost always). The three eight-item subscales each have adequate reliability with Cronbach’s α of .65 for anger out, .81 for the anger control, and .79 for anger in. The Anger Expression Scale was administered after lunch around 1:00 PM.
The Cook-Medley Hostility Scale (CMHOS; Cook and Medley, 1954) measured dispositional tendencies toward suspiciousness, resentment, and cynical mistrust. Participants rated 50 statements related to those psychological characteristics as either true or false. Higher score indicated greater hostility. The Cronbach’s α of the scale was .82. The CMHOS was administered at 7:15 AM on the second day of the admission at the GCRC.
The Beck Depression Inventory-Short Form (BDI) was used to evaluate depressive symptomatology (Beck et al. 1988b). Respondents described how they had been feeling in the past week. The 13 items related to the severity of affective and cognitive depressive symptoms. A higher score indicated more depressive symptoms. The BDI has good internal reliability; the Cronbach’s α was .79. The BDI was completed before blistering, around 7:15 AM.
The Beck Anxiety Inventory (BAI) assessed both cognitive and physiological anxiety symptoms (Beck et al. 1988a). Participants indicated the extent that they were bothered by anxiety symptoms using a four-point likert scale (0 =not at all bothered to 3 = severely bothered). Higher scores indicated higher levels of anxiety symptoms. The scale has good internal consistency with Cronbach’s α of .83. The BAI was completed before blistering around 7:15 AM.
The Positive and Negative Affect Schedule (PANAS) assessed positive and negative mood state (Watson et al., 1988). Participants rated the extent that they experienced 10 negative and 10 positive affective states in the last two hours on a 5-point likert scale. Higher score reflected greater intensity of affect. The two subscales have good internal consistency with a Cronbach‘s α ranging from 0.71–.0.80 for the negative affect subscale and 0.74–0.80 for the positive affect subscale. The PANAS was administered after each relaxation session.
The Interpersonal Support Evaluation List (ISEL; Cohen, Mermelstein, Kamarck, Hoberman, 1985) was used to measure perceived social support. This 40-item scale assesses four types of social support: self-esteem, appraisal, tangible, and belonging. Items are rated on a four-point likert scale (1=definitely false to 4 = definitely true) with higher scores reflecting higher perceived social support. The Cronbach’s α of the scale was .93. The ISEL was administered after lunch around 1:00 PM.
Health-related behaviors were assessed according to previous recommendations (Kiecolt-Glaser and Glaser, 1988). The amount of alcohol consumption, and the number of hours of physical exercise in the past week were evaluated using single-item measures. The Pittsburgh Sleep Quality Index was used to assess sleep quality and disturbances in the past week (Buysse et al., 1989). In addition, on admission to the GCRC, nurses measured blood pressure, and weight and height. Information regarding participants’ nutritional status was assessed using albumin assay.
2.5 Suction Blister Model
A commercial suction blister device (Neuro Probe, Cabin John, MD) was used to separate the dermal-epidermal junction, creating 8 small 8 mm sterile blisters, as previously described (Glaser et al., 1999; Kuhns et al., 1992). In order to assess the early phase of the inflammatory response to wounding in vivo, the tops of the blisters were removed with sterile scissors, a plastic template was placed over the blister sites, and the chambers were filled with approximately 1 ml of chamber fluid composed of 70% autologous serum in Hank’s balanced salt solution. Chamber fluid from 3 wells was extracted and pooled for the 4 and 7 hours samples, and 2 wells were pooled for the 22 hours sample. The pooled samples were analyzed for cytokines levels and cell numbers.
Measurement of the rate of transepidermal water loss (TEWL) through human skin provides a noninvasive method to monitor changes in the stratum corneum barrier function of the skin. TEWL was measured using a vapor pressure gradient estimation method. TEWL decreased as the barrier was restored; thus, monitoring of TEWL over time allowed objective evaluation of wound healing (Smit et al., 1990). After subtracting the average control values from the daily measurement, the standard of healing was based on reaching 90% of the day 1 measures. A computerized evaporimetry instrument, the DermaLab® (CyberDERM, Media, PA) was used to measure TEWL, following established procedural guidelines (Pinnagoda et al., 1990).
2.6 Immunological and Endocrinological Assays
Chamber fluid IL-1α, IL-1β, IL-6, IL-8, and TNF-α were assayed using Quantikine High Sensitivity Immunoassay kits (R&D Systems, Minneapolis, Minn), per kit instructions, as described elsewhere (Kiecolt-Glaser et al., 2003). Samples were run undiluted in duplicate.
Salivary cortisol was assayed using the Cortisol Coat-A-Count RIA (Diagnostic Products Corporation, Los Angeles, CA). The saliva sample obtained from a dental cotton roll was lyophilized and reconstituted at a 5-fold higher concentration for the afternoon and evening values to fall within detection limits. Cortisol samples were assayed in duplicate. This procedure had excellent sensitivity and specificity, with intra-assay variation of 4.3% and inter-assay variation of 5.2%.
2.7 Statistical Analysis
An analysis of covariance controlling for age of the participants was performed to examine difference in the rate of healing between the relaxation intervention and the control groups. T-tests were subsequently used to evaluate group differences in anger expression. Following the Ebrecht et al., (2004) analytic strategy, fast and slow healing groups were created by a median split on the number of days to heal variable. Participants whose wound reached the 90% return to TEWL baseline criterion within four days were considered “fast healers” while participants who took more than four days to heal were considered “slow healers.” According to that criterion, 46 (46.9%) participants were fast healers and the remaining 52(53.1%) participants were slow healers. The healing status at day 4 was used as the main outcome variable.
Logistic regression was used to investigate whether anger expression predicted healing status at day 4. A goodness-of-fit χ2 was used to compare the log-likelihood of a model including the anger expression variable to a model excluding it. The Wald statistic was used to investigate the contribution of individual predictors within each model to the prediction of the healing status at day 4. Logistic regressions were also performed to investigate whether anger expression predicted wound healing over and above differences in hostility, negative affectivity, social support, and health behaviors. Multiple regression analyses were conducted to determine whether anger expression was related to cortisol secretion. The cortisol area under the curve (AUC) was calculated according to the trapezoid formula proposed by Pruessner et al. (2003). The cortisol AUC “ground” was calculated using saliva samples from 7 AM to 10 PM, while the cortisol AUC “increase” was computed using the samples from 7 AM to 10:30 AM. Cortisol slope were computed by regressing the log-transformed cortisol values on the time of the samples collection, after dropping the 13h sample (lunch rise). The slope of the regression line was used to represent each participant diurnal variation in cortisol. A mediation model was tested according to the Baron and Kenny (1986) procedure. However, the logistic regression coefficients were standardized as recommended by MacKinnon and Dwyer (1993) when testing mediation with a dichotomous outcome variable. A Sobel test using the standardized regression coefficients was used to the test the significance of the indirect effect. Repeated-measures regression models were also performed to investigate whether anger expression influenced the production of cytokines at the wound site over time. The interaction term tested the hypothesis of a different pattern of change in cytokines production among participants with different types of anger expression. Similarly, the associations between cortisol secretion during the GCRC admission and the production of cytokines over time were examined using repeated-measures regression models.
3. Results
Psychological, endocrinological, and immunological data were collected from 100 participants. Of those, two participants were excluded from subsequent analyses because their body mass index was more than three standard deviations above the mean. Fifty-seven participants were randomized to the relaxation intervention while 41 were assigned to the control group. No sex differences were found in the rate of healing, F (1,97) = 0.31, p = 0.86. Psychological characteristics and health behaviors of the participants can be found in Table 2. Inter-correlations between the psychological variables are reported in Table 3.
Table 2.
Psychological Characteristics and Health Behaviors of the Study Participants
| Mean | SD | Range | |
|---|---|---|---|
| Anger Out | 13.55 | 2.73 | 8–21 |
| Anger Control | 25.20 | 4.14 | 16–32 |
| Anger In | 15.11 | 3.77 | 8–26 |
| Hostility | 14.47 | 6.75 | 2–36 |
| Depression | 2.45 | 3.04 | 0–15 |
| Anxiety | 2.18 | 2.36 | 0–14 |
| Negative Affect | 10.95 | 1.22 | 10–16.17 |
| Social Support | 94.53 | 14.53 | 58–120 |
| Body Mass Index | 25.03 | 3.17 | 18.13–32.15 |
| Exercise (hours/week) | 3.34 | 1.88 | 0–7 |
| Alcohol use (# of drink/week) | 1.63 | 2.94 | 0–10 |
| Sleep Disturbances | 1.34 | 0.48 | 1–12 |
Table 3.
Zero-Order Correlations Between Psychological Variables
| Anger Out | Anger Control | Anger In | Depression | Anxiety | Negative Affect | Hostility | Social Support | |
|---|---|---|---|---|---|---|---|---|
| Anger-Out | - | −.45** | .18 | .08 | .03 | .01 | .40** | −.11 |
| Anger-Control | - | −.07 | .03 | −.04 | −.04 | −.32** | .03 | |
| Anger-In | - | .36** | .06 | .22* | .36** | −.39** | ||
| Depression | - | .25* | .32** | .16 | −.44** | |||
| Anxiety | - | .52** | .22* | −.04 | ||||
| Negative Affect | - | .22* | −.16 | |||||
| Hostility | - | .28** | ||||||
| Social Support | - |
p < .05
p < .01
3.1 Relaxation and Wound Healing
An ANCOVA controlling for age was performed to compare the rate of healing between the two groups. The relaxation and the control group did not differ on the speed of healing, F(1, 97) = 0.39, p = 0.53. Since the intervention had no impact on wound healing, mediation analysis involving inflammatory mediators at the wound site was not be performed.
3.2 Anger expression and Wound Healing
No significant differences in anger expression were observed between the intervention and the control group (all p’s > 0.1). Inclusion of group membership did not significantly alter the impact of anger expression on wound healing; consequently those results are not reported. In order to test our main hypothesis we performed a direct logistic regression with healing status at day 4 as the outcome variable and the three anger expression variables as predictors. A test of the full model with all three anger predictors against a constant-only model was statistically significant, χ2(4, N= 98) = 8.00, p = 0.046, indicating that the anger expression variables distinguished between fast and slow healers. According to the Wald criterion, only anger control predicted the healing status at day 4, z = 7.14, p = 0.008, while anger out, z = 1.10, p = .294, and anger in, z = 0.002, p = .963, were not significantly associated with healing status. Results showed that higher levels of anger control were associated with a higher likelihood of being healed at day 4. Specifically, a one-point increase in anger control increased the odds of being categorized as a fast healer by 1.18 (95% CI: 1.05 – 1.33). Nagelkerke R2 indicated that anger control accounted for 9 % of the variance in healing status. Figure 1 depicts the percentage of participants healed at each follow-up measurement in function of their anger control levels.
Fig 1.
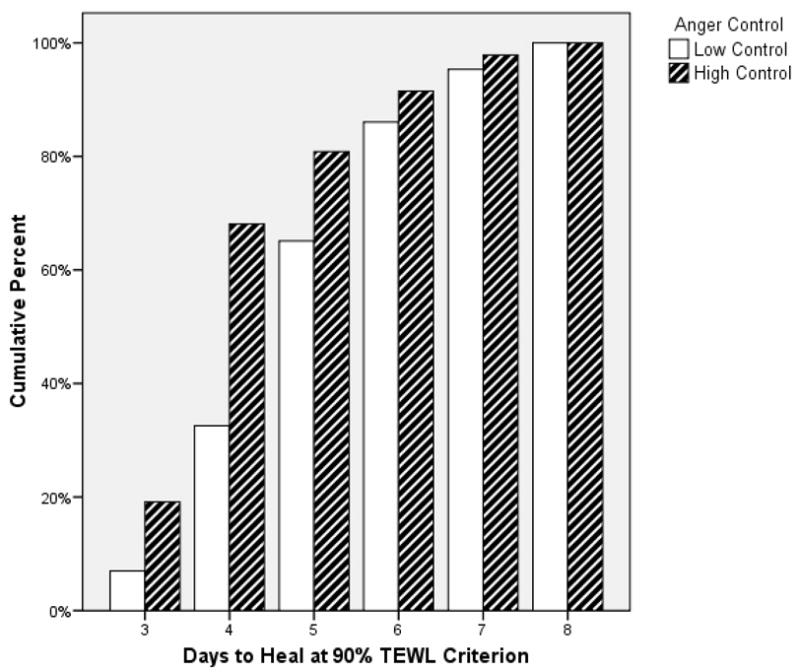
Days to Heal in Function of Anger Control Levels
Subsequently, a sequential logistic regression was carried out to determine if the anger control variables predict healing status at day 4 above and beyond the contribution of age, sex, and body mass index. The comparison of log-likelihood ratios for the model with and without the socio-demographic variables showed that the addition of the anger control variable significantly improved the performance of the model, χ2(4, N= 98) = 9.98, p = 0.041. Inclusion of the other anger expression variables rendered the model marginally significant, χ2(6, N= 98) = 11.43, p = 0.076. Nevertheless, the Wald statistic showed that anger control was still a significant predictor of the healing status at day 4, z = 7.45, p = 0.006, while anger out, z = 1.34, p = .247, and anger in, z = −0.025, p = .975, did not significantly contribute to the prediction of the healing status at day 4. The impact of sex differences on anger expression was further assessed by comparing the log-likelihood ratios of a model with and without sex by anger expression style interaction terms. Results showed that the model containing the anger expression by sex interaction terms did not significantly predict the healing status at day 4, χ2(9, N= 98) = 14.0, p = 0.106. Wald statistics revealed that neither the interaction of sex by anger-control, z = 2.65, p = 0.103 anger-out, z = 0.43, p = 0.51, or anger-in, z = 0.36, p = 0.55 were significant. Since the anger out and anger in variables did not appear to make a significant contribution to the prediction of the healing status, only anger control was included in subsequent analyses.
3.3 Potential Confounds and Psychosocial Mechanisms Linking Anger Control and Healing
Since the impact of anger control on health outcomes sometimes disappeared when dispositional hostility is taken into account (Burg et al. 2004), a sequential logistic regression was performed to examine whether anger control was related to healing over and above the contribution of hostility. Age, sex, and bmi were entered in the first step, hostility was entered in the second step, and anger control was included in the third step. Comparison of the log-likelihood ratios showed that the model including anger control was significant against a model including hostility only, χ2(6, N= 98) = 10.38, p = 0.035. The Wald statistic showed that only anger control was significantly associated with the healing status at day 4, z = 7.05, p = 0.008.
In addition, we evaluated the possibility that the association between anger control and healing was due to an overall difference in negative affect. In order to obtain the best estimate of the levels of negative affect experienced by the participants, the 6 PANAS measurement obtained during the GCRC admission were averaged. A sequential logistic regression was then used to compare a model including negative affect, depression, and anxiety to a model with those variables and anger control. Four participants were excluded from this analysis because of missing data on negative affect variables. Comparison of the log-likelihood ratios showed that the model including anger control with the negative affect variables was significant against a model including only the negative affect variables, χ2(4, N= 94) = 12.34, p = 0.015. The Wald criterion showed that within this model, only the anger control variable was significantly associated with healing status at day 4, z = 8.3, p = 0.004.
Previous studies have found that the impact of anger expression on health outcomes was moderated by social support (Angerer et al. 2000). In order to the test this potential psychosocial mechanism, a sequential logistic regression was performed. Comparison of the log-likelihood ratios showed that the model including the anger control by social support interaction terms was not significant against a model including only anger control and social support, χ2(6, N= 94) = 0.87, p = 0.35.
To rule out the possibility that the association between anger control and healing was explained by differences in health behaviors, a second sequential logistic regression was conducted. Sleep disturbances, amount of physical activity, and alcohol consumption in the past week were entered in the first step; the anger control variable was entered in the second step. Four participants were excluded from this analysis because of missing data on health behavior variables. The comparison of the log-likelihood ratios of the model with and without the health behavior variable, showed that anger control still uniquely predicted the healing status at day 4, χ2(4, N= 94) = 10.57, p = 0.032, above and beyond the variance accounted for by sleep, exercise, and alcohol consumption. The Wald criterion confirmed that within this model, only the anger control variable was significantly associated with healing status at day 4, z = 6.11, p = 0.013.
3.4 Physiological Mechanisms Linking Anger Control and Wound Healing
The physiological mechanism by which anger control influenced wound healing was also investigated. A regression analysis investigated the relationship between anger control and the total cortisol secretion across the day of the admission at the GCRC. Using age, sex, BMI, and sleep disturbances as covariates, anger control was not significantly associated with the cortisol AUC from 7:00 AM to 10:00 PM, β = 0.107, p = 0.52. A sex by anger control interaction term did not significantly predict the AUC, β = 0.47, p = 0.49. We also investigate whether anger control was associated with the diurnal variation in cortisol. A regression analysis showed that anger control was not associated with the diurnal cortisol slope, β = 0.06, p = 0.55. A sex by anger control interaction term did not significantly predict the cortisol slope, β = 0.11, p = 0.88.
The anticipation of the blistering procedure as well as the wounding manipulation itself appeared to have been experienced as a mild stressor by the participants. Indeed, they exhibited an increase in blood pressure at admission, compared to discharge, F(1, 94)= 40.19, p < 0.001, and higher levels of negative affect during the blistering compared to the rest of the admission, F(4, 94) = 22.96, p < 0.001. A regression analysis was conducted to evaluate whether anger control was associated to the cortisol reactivity to the stress induced by the blistering procedure. Using age, sex, BMI, and sleep disturbances as covariates, anger control was significantly associated with the cortisol AUC increase, β = 0.24, p = 0.04, adjusted R2 = 0.045. A sex by anger control interaction term did not significantly predict cortisol secretion during the blistering period, β = 0.36, p = 0.61.
The mean cortisol value at each measurement occasion was plotted using the dichotomous anger control variable (see Figure 2). Since the first data point appeared to be the highest, the AUC ‘increase’ provided an index of decrease in cortisol secretion as described by Pruessner et al. (2003). Visual inspection of the cortisol slopes revealed that participants with higher levels of anger control had a smaller index of decrease due to the fact that their initial cortisol values were lower. Therefore, in this context a smaller index of decrease indicated smaller total cortisol secretion during the blistering period.
Fig 2.
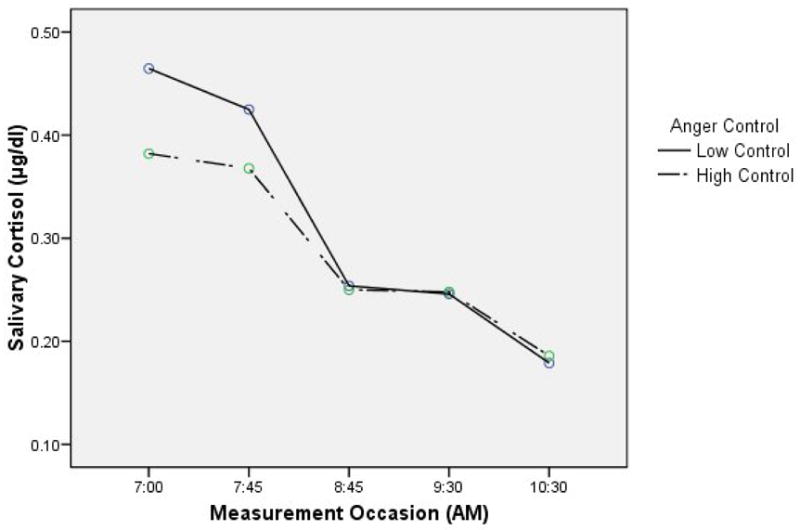
Area Under the Curve ‘Increase’ During the Blistering Procedure
A direct logistic regression assessed the relationship between cortisol AUC increase during the blistering period and the healing status at day 4. A test of the full model with the cortisol predictor against a constant-only model was statistically significant, χ2(1, N= 76) = 5.77, p = 0.016. Results indicated that participants who exhibited lower production of cortisol during the blistering period were more likely to be healed at day 4. Nagelkerke R2 indicates that the AUC increase accounts for 9.8% of the variance in Healing Status.
A mediation model with anger control as the predictor variable, cortisol AUC increase as the mediator, and healing status at day 4 as the outcome was tested. Inclusion of both anger control and cortisol AUC increase in the same model with age, sex, and BMI as covariates resulted in a significant model against a model including the covariates only, χ2(5, N= 76) = 11.36, p = 0.045, Wald criterion shows that anger control was no longer a significant predictor, z = 3.64, p = .057 while the mediator, cortisol AUC increase, was still a significant predictor, z = 3.99, p = .046. The small reduction in the β value suggested a partial mediation of the effect of anger control on healing status at day 4 by the cortisol secretion during the blistering period. The Sobel test of the indirect effect using standardized logistic regression coefficient was marginally significant (p= 0.08), confirming that the effect of anger control on wound healing was only partially mediated by the cortisol response to blistering-induced stress.
Repeated-measures regression models were also performed to investigate if changes over time in the production of cytokines at the wound site were associated with anger control. Changes in IL-1α, IL1-β, IL-6, IL-8, and TNF-α at the wound site were examined at 4, 7, and 22 hours post-blistering. The repeated-measures regression models were performed with the 72 participants for whom cytokines data were collected. Using a Greenhouse-Geisser correction, the interaction between changes in cytokines production over time and anger control was not significant for any of the cytokines (all p’s > 0.19). The possibility that the cortisol secretion during the blistering period was associated with changes in cytokine production over time was also examined. Repeated-measures regression models revealed that the cortisol secretion during blistering was not significantly associated with changes in any of the cytokines assayed (all p’s > 0.68). Repeated-measures regression models including a sex by cortisol secretion interaction were also not significantly associated with local production of cytokines (all p’s > 0.38).
4. Discussion
This study addressed relationships between anger expression and wound healing. Anger control was a significant predictor of the healing status at day 4, while anger out and anger in were not related to the speed of healing. Anger control predicted healing over and above differences in hostility, negative affectivity, social support, and health behaviors. Individuals displaying less ability to regulate the expression of their anger secreted more cortisol in response to the stress associated with the blistering procedure. Greater cortisol production was in turn associated with delayed healing. Changes in proinflammatory cytokines at the wound site were not associated with anger expression.
The impact of anger control on wound healing has clinical relevance. Individuals with low control over the expression of their anger were 4.2 times more likely to take more than 4 days to heal, compared to those with higher levels of anger control. Such stress-induced delays in healing could increase the susceptibility to infection at the wound site, a process that fuels further decrease in the speed of repair (Rojas et al. 2002; Robson, 1997). In fact, longer hospitalization stay and an increased number of post-surgery complications have been observed in individuals reporting higher levels of anger (Sharma et al. 2007; Stengrevics et al. 1996). Those results provide evidence that the ability to regulate the expression of one’s anger is a psychological characteristic that appears to have a clinically relevant impact on wound repair.
Contrary to our hypothesis, anger out and anger in were not significantly associated with the speed of healing. In our sample, participants had low anger out scores ranging from 8 to 21, while the highest possible value of this subscale was 32. This suggests that a range restriction problem may have hindered our ability to find a meaningful relationship between anger out and healing. On the other hand, our results show that it was not the expression of anger per se, but the control over the anger that was related to wound repair. Deffenbacher and colleagues (1996) found that higher levels of anger control were related to a decreased frequency and intensity of feelings of anger as well as less severe anger-related consequences. Also, individuals with lower control over the expression of their anger appear to use less efficient coping strategies leading to more psychological distress and poorer quality of life than those with higher anger control (Diong et al. 2005; Julkunen and Ahlstrom, 2006). In fact, cardiac patients with poorer anger control were more likely to experience a silent ischemia during a mental stress task, compared to patients with higher anger control (Burg et al. 1993). Therefore, individuals with low levels of anger control seem to exhibit more psychological and physiological reactivity following exposure to a stressful event.
Participants appeared to have experienced the anticipation of the blistering as well as the blistering itself as a mild, transient stressor. Indeed, higher blood pressure and higher PANAS negative affect score were observed during this period, compared to the rest of the admission at the GCRC. The cortisol response to this mild stress was greater among individuals exhibiting less control over the expression of their anger than participants who reported higher levels of anger control. These data are consistent with evidence that cortisol can alter the course of healing (Gupta et al. 1999). The first 24 hours following wounding appears to be a critical period during which dysregulation of the repair process leads to subsequent delays in healing (Kirsner and Eaglstein, 1993). Indeed, elevated cortisol secretion the day following a punch biopsy was related to slower healing, while glucocorticoid production 13 days later was not (Ebrecht et al. 2004). Thus, it is not surprising that the increase in cortisol reactivity on the day of the blistering was related to the speed of healing. However, the mediation analysis revealed that factors other than the cortisol secretion also contribute to impact of anger control on wound healing.
Potential confounding factors that could have explained the relationship between anger control and wound healing were examined. The results indicated that anger control remained a significant predictor of the healing status at day 4 when differences in hostility and negative affect were taken into account. Similarly, the association between anger control and wound repair persisted even when health behaviors such as sleep, exercise, and alcohol use were controlled, suggesting that anger control influenced healing over and above the impact of health behaviors and general negative affectivity.
Another potential pathway by which the ability to regulate one’s anger can influence wound healing is through the disruption of interpersonal relationships. In contrast to fear and sadness, the expression of anger has been associated with social maladjustment (Kubany et al. 1995). Individuals who lack control over their anger are frequently described by others as abrasive, confrontational, and opinionated (Deffenbacher, 1993). In fact, angrier individuals report fewer and less satisfying social supports, while individuals displaying higher anger control present better personal relationships (Dalhen and Martin, 2005; Diong et al. 2005). However, in this study the impact of anger control on wound healing did not appear to be moderated by social support.
A previous study from our laboratory showed that hostile behaviors during the discussion of a marital disagreement delayed wound healing in part by down-regulating the production of proinflammatory cytokines at the wound site (Kiecolt-Glaser et al. 2005). In the present study, anger control was not associated with the local production of cytokines. Key methodological differences might explain the differences in the results observed in the two studies. In the marital interaction study, actual behaviors observed by independent judges during the marital discussion were related to the rate of healing while in the current study perception of one’s ability to control the expression his/her anger was related to healing. The physiological mechanism linking a personality trait to wound healing is likely to be different from the pathways linking actual behaviors and healing. Furthermore, the magnitude and the type of stressors differed between the two studies. In both studies, the blistering procedure generated mild stress for the participants. However, in the marital interaction study, the participant underwent the additional stress of the discussion of a marital conflict. Stressors involving a social-evaluative component lead to larger cortisol secretion and longer recovery (Dickerson & Kemeny, 2004), changes that are more likely to fuel disruption in local cytokine production.
This study was part of a larger project examining the impact of a relaxation intervention on wound healing. Our hypothesis that the relaxation intervention would lead to faster healing was not supported. This is surprising considering that numerous studies have found that relaxation before surgery leads to better post-operative outcomes (Montgomery et al., 2002). A floor effect problem may have hindered our ability to detect meaningful effects of our intervention. Patients undergoing surgery in clinical settings experience many sources of stress and concerns: risks associated with surgery, hospital stay, and pain (Walker, 2002). In contrast, participants in our study received a very superficial wound executed in a controlled environment. Although our participants reported mild stress associated with the blistering procedure, the magnitude of the stressors is far less than what a patient would experience in a clinical setting. Hence, the null effect of the intervention might result from the overall low levels of stress experienced by the participants during the study impeding the possibility that significant reduction in stress occurred as a result of the intervention (Linden and Satin, 2007). In addition, there is some evidence that participants receiving the relaxation intervention provided by an experienced therapist were more likely to be healed at day 4 compared to junior therapists, χ2 (1, N = 42) = 3.5, p = 0.065.
Limitations of this study include the restricted inclusion of participants from diverse ethnic backgrounds. Replication of this study will be necessary to generalize our results to a non-Caucasian population. This is especially important since the social desirability of different types of anger expression depends upon the socio-cultural context in which it occurs (Sharkin, 1996). In addition, accrual of participants with a wider range of anger expression would allow a better test of the association between anger out, anger in, and wound healing.
In sum, this is the first study showing that difficulty in anger regulation can lead to delayed healing. Furthermore, an exacerbated cortisol response to stress appears to explain the relationship between lower anger control and wound repair, although other physiological pathways may mediate the association between anger regulation and healing.
The impact of anger control appears to have a clinically relevant impact on wound repair. Fortunately, successful therapeutic strategies have been designed to help individuals improve their control over the expression of their anger (Del Vecchio and O’Leary, 2004). Future studies should test whether anger control therapy can increase the rate of healing among individuals having difficulty regulating this negative emotion.
Acknowledgments
Work on this article was supported by a Doctoral Training Award from the Fonds de la Recherche en Santé du Québec, NIH Grant DE13749, NIH General Clinical Research Center Grant MO1-RR0034, and Comprehensive Cancer Center Grant CA16058.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer P, Siebert U, Kothny W, Muhlbauer D, Mudra H, von Schacky C. Impact of social support, cynical hostility and anger expression on progression of coronary athrosclerosis. J Am Coll Cardiol. 2000;36:1781–1788. doi: 10.1016/s0735-1097(00)00944-x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988a;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988b;8:77–100. [Google Scholar]
- Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22:440–448. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- Burg MM, Lampert R, Joska T, Batsford W, Jain D. Psychological traits and emotion-triggering of ICD schock-terminated arrhythmias. Psychosom Med. 2004;66:898–902. doi: 10.1097/01.psy.0000145822.15967.15. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001;63:216–220. doi: 10.1097/00006842-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- Dahlen ER, Martin RC. The experience, expression, and control of anger in perceived social support. Pers Individ Dif. 2005;39:391–400. [Google Scholar]
- Deffenbacher JL. General anger: Characteristics and clinical implications. Psicologia Conductal. 1993;1:49–67. [Google Scholar]
- Deffenbacher JL, Oetting ER, Lynch RS, Morris CD. The expression of anger and its consequences. Behav Res Ther. 1996;34:575–590. doi: 10.1016/0005-7967(96)00018-6. [DOI] [PubMed] [Google Scholar]
- Del Vecchio T, O’Leary DK. Effectiveness of anger treatments for specific anger problems: A meta-analytic review. Clin Psychol Rev. 2004;24:15–34. doi: 10.1016/j.cpr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries CA. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical intergration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diong SM, Bishop GD, Enkelmann HC, Tong EMW, Why YP, Ang JCH, Khader M. Anger, stress, coping, social support and health: Modelling the relationships. Psychol Health. 2005;20:467–495. [Google Scholar]
- Ebrecht M, Hextall J, Kirtley L-G, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004;29:798–809. doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- Eckhardt C, Norlander B, Deffenbacher J. The assessment of anger and hostility: a critical review. Agress Violent Beh. 2004;9:17–43. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- Gupta A, Jain GK, Raghubir R. A time course study for the development of an immunocompromised wound model, using hydrocortisone. J Pharmacol Toxicol Methods. 1999;41:183–187. doi: 10.1016/s1056-8719(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Hogan BE, Liden W. Curvilinear relationships of expressed anger and blood pressure in women but not in men: Evidence from two samples. J Psychosom Res. 2005;59:97–102. doi: 10.1016/j.jpsychores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Ishihara S, Makita S, Imai M, Hashimoto T, Nohara R. Relationship between natural killer activity and anger expression in patients with coronary heart disease. Heart Vessels. 2003;18:85–92. doi: 10.1007/s10380-002-0687-4. [DOI] [PubMed] [Google Scholar]
- Julkunen J, Ahlstrom R. Hostility, anger, and sense of coherence as predictors of health-related quality of life. Results of an ASCOT substudy. J Psychosom Res. 2006;61:33–39. doi: 10.1016/j.jpsychores.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychological stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery: Perspectives from psychoneuroimmunology. Am Psychol. 1998;53:1209–1218. doi: 10.1037//0003-066x.53.11.1209. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsner RS, Eaglstein WH. The wound healing process. Dermatol Clin. 1993;11:629–640. [PubMed] [Google Scholar]
- Kubany ES, Bauer GB, Muraoka MY, Richard DC, Read P. The impact of labelled anger and blame in intimate relationship. J Soc Clin Psychol. 1995;14:53–60. [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuhns DB, DeCarlo E, Hawk DM, Gallin JI. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest. 1992;89:1734–1790. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden W, Satin JR. Avoidable pitfalls in behavioral medicine outcome research. Ann Behav Med. 2007;33:143–147. doi: 10.1007/BF02879895. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–158. [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- Miller GE, Dopp JM, Myers HF, Felten SY, Fahey JL. Psychosocial predictors of natural killer cell mobilization during marital conflict. Health Psychol. 1999;18:262–271. doi: 10.1037//0278-6133.18.3.262. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, David D, Winkel G, Silverstein JH, Bovbjerg DH. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth Analg. 2002;94:1639–1645. doi: 10.1097/00000539-200206000-00052. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR, Kinsinger D, Antoni MH, Molton I, Gonzalez JS, Fletcher MA, Roos B, Carver CS, Schneiderman N. Anger suppression mediates the relationship between optimism and natural killer cell cytotoxicity in men treated for localised prostate cancer. J Psychosom Res. 2006;60:423–427. doi: 10.1016/j.jpsychores.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermatitis. 1990;22:164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinolgy. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77:637–650. doi: 10.1016/s0039-6109(05)70572-7. [DOI] [PubMed] [Google Scholar]
- Rojas I, Padgett DA, Sheridan JF, Marucha PT. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16:74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- Scanlan JM, Vitaliano PP, Zhang J, Savage M, Ochs HD. Lymphocyte proliferation is associated with gender, caregiving, and psychosocial variables in older adults. J Behav Med. 2001;24:537–559. doi: 10.1023/a:1012987226388. [DOI] [PubMed] [Google Scholar]
- Sharkin BS. Understanding anger: Comment on Deffenbacher, Oetting et al., (1996), Deffenbacher, Lynch et al., (1996), and Kopper and Epperson (1996) J Couns Psychol. 1996;43:166–169. [Google Scholar]
- Sharma A, Sharp DM, Walker LG, Monson JRT. Patient personality predicts postoperative stay after colorectal cancer resection. Colorectal Dis. doi: 10.1111/j.1463-1318.2007.01287.x. In Press. [DOI] [PubMed] [Google Scholar]
- Smit HA, Pinnagoda J, Tupker RA, Burema J, Coenraads PJ, Nater JP. Variability in transepidermal water loss of the skin: Evaluation of a method to assess susceptibility to contact dermatitis in epidemiological studies. Int Arch Occup Environ Health. 1990;62:509–512. doi: 10.1007/BF00381181. [DOI] [PubMed] [Google Scholar]
- Smith TW. Concepts and methods in the study of anger, hostility, and health. In: Siegman AW, Smith TW, editors. Anger, Hostility, and the Heart. Lawrence Erlbaum; Hillsdale, NJ: 1994. pp. 23–42. [Google Scholar]
- Spielberger CD, Jacobs G, Russell S, Crane RS. Assessment of Anger: The State-Trait Anger Scale. Adv Pers Assess. 1983;2:159–187. [Google Scholar]
- Spielberger CD. Psychological Assessment Resources. Odessa, FL: 1988. State-Trait Anger Expression Inventory professional manual. [Google Scholar]
- Stengrevics S, Sirois C, Schwartz C, Friedman R, Domar A. The prediction of cardiac surgery outcome based upon preoperative psychological factors. Psychol Health. 1996;11:471–477. [Google Scholar]
- Walker JE. Emotional and psychological preoperative preparation in adults. Br J Nurs. 2002;11:567–575. doi: 10.12968/bjon.2002.11.8.10166. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Phil D, Cropley M, Griffith J, Kirschbaum C. Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosom Med. 2000;62:286–292. doi: 10.1097/00006842-200003000-00022. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Sex differences in the relation of depressive symptoms, hostility, and anger expression to indices of glucose metabolism in nondiabetic adults. Health Psychol. 2006;25:484–492. doi: 10.1037/0278-6133.25.4.484. [DOI] [PubMed] [Google Scholar]
- Surber C, Schwarb FP, Smith EW. Tape-stripping technique. In: Bronaugh RL, Maibach HI, editors. Percutaneous Absorption: Drugs-Cosmetics-Mechanisms-Methodology. Marcel Dekker; New York: 1999. pp. 395–409. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]


