Abstract
We have examined the role played by protein kinase A (PKA) in vesicle-mediated protein transport from the trans-Golgi network (TGN) to the cell surface. In vivo this transport step was inhibited by inhibitors of PKA catalytic subunits (C-PKA) such as the compound known as H89 and a myristoylated form of the inhibitory peptide sequence contained in the thermostable PKA inhibitor. Inhibition by H89 occurred at an early stage during the transfer of vesicular stomatitis virus G glycoprotein from the TGN to the cell surface. Reversal from this inhibition correlated with a transient increase in the number of free coated vesicles in the Golgi area. Vesicle budding from the TGN was studied in vitro using vesicular stomatitis virus-infected, permeabilized cells. Addition to this assay of C-PKA stimulated vesicle release while it was suppressed by PKA inhibitory peptide, H89, and antibody against C-PKA. Furthermore, vesicle release was decreased when PKA-depleted cytosol was used and restored by addition of C-PKA. These results indicate a regulatory role for PKA activity in the production of constitutive transport vesicles from the TGN.
Transport of newly synthesized proteins along the exocytic pathway is mediated by small vesicular carriers (1, 2). A set of proteins collectively known as the transport machinery is responsible for the formation and consumption of vesicles. These include receptor proteins that sort and concentrate cargo molecules at the budding site, coat proteins that catalyze budding from the donor compartment, and proteins responsible for targeting and fusion with the acceptor membrane (1). Recent evidences indicate that the transport machinery is regulated by specific phosphorylating activities that in this way modulate intracellular traffic. Thus, phosphatidylinositol-3 kinase has been shown to be required for transfer of hydrolases from the Golgi to either the yeast vacuole (3) or the mammalian lysosomes (4, 5). Release of secretory vesicles from the trans-Golgi network (TGN) of endocrine cells has also been shown to be regulated by tyrosine phosphorylation (6). Both protein kinase A (PKA) and protein kinase C (PKC) activities have been reported to control particular vesicle-mediated transport steps such as constitutive (7–11) and regulated exocytosis (12–15) and transcytosis (16, 17). Whereas the effects derived from the activation/inhibition of these different kinases on transport are well documented little information is available about their mechanism of action. PKC activity has been described to stimulate association of ADP ribosylation factor and coatomer proteins to Golgi membranes (18) but recent data indicate that its phosphorylating activity is not required for vesicle budding from the TGN (19). Therefore, it is uncertain at present in which way protein kinases control transport activities. We have recently described that PKA differentially regulates constitutive protein transport along the exocytic pathway with transport from the TGN to the cell surface being strictly dependent on PKA activity (20). We now have extended these studies to the characterization of the site of action of PKA. The results indicate that vesicle budding from the TGN requires PKA activity.
MATERIALS AND METHODS
Materials.
Bovine brain cytosol was prepared as described (21) and dialyzed extensively against 25 mM Tris⋅HCl, pH 8.0, 50 mM KCl, and 1 mM DTT. The preparation was centrifuged for 1 h at 100,000 × g (SW60 rotor, Beckman Instruments) to remove aggregates; aliquots were frozen and maintained at −70°C. Before being used cytosolic proteins were transferred to 25 mM Hepes-KOH, pH 7.0, containing 25 mM KCl, 2.5 mM MgCl2 by application to a Econo-PacI0DG Bio-Rad column. To deplete PKA, 0.5 ml cytosol (≈4 mg/ml) was incubated with cAMP-agarose (Sigma) for 1 h at 4°C, packed into a 2.5 ml column and the flow-through fraction collected. The following antibodies were used: 8G5F11 (clone I1) monoclonal antibody against the extracytoplasmic domain of vesicular stomatitis virus G glycoprotein (VSV-G) (22) was kindly provided by D. S. Lyles (Wake Forest University, NC); P5D4 mAb against the C terminus of VSV-G (23) was purchased from Sigma; rabbit polyclonal IgG against PKA catalytic subunit (C-PKA) was obtained from Santa Cruz Biotechnology; and antisera recognizing TGN38 (24) and α-1,2-mannosidase II (25) were obtained from G. Banting (University of Bristol, Bristol, U.K.) and M. G. Farquhar (University of California, San Diego), respectively. C-PKA, PKA inhibitory peptide (PKI), apyrase, neuraminidase, protein G-Sepharose were purchased from Sigma. Myristoyl-PKI was from Quality Control Biochemicals (Hopkinton, MA), N-[2-(-p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H89) from Calbiochem, and endoglycosidase H and proteinase K from Boehringer Mannheim.
Cell Culture and Virus Infection.
Normal rat kidney cells were maintained in monolayer culture in DMEM containing 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Confluent cultures were infected at 37°C with VSV at a multiplicity of 25–30 plaque-forming units per cell in serum-free medium for 45 min (26). Cells were incubated in complete medium for 3 h before start the experiments.
Metabolic Labeling and VSV-G Immunoprecipitation.
Cells cultured on 35-mm dishes were used. They were incubated at 37°C in methionine- and cysteine-free medium for 15 min and radiolabeled for 5 min with 25 μCi Tran35S-label (1 Ci = 37 GBq). Cells were rinsed and chased at 20°C in complete medium containing 1.5 mg/ml of both unlabeled methionine and cysteine. Immunoprecipitation of VSV-G from the cell surface was performed with 8G5F11 antibody as described (20). Intracellular VSV-G was immunoprecipitated with P5D4 antibody (20). Immunocomplexes were purified with protein G-Sepharose and analyzed by SDS/PAGE.
Vesicle Release Assay from Permeabilized Cells.
The assay described by Müsch et al. (27) was used with few modifications. Cells cultured on 100-mm dishes were infected, radiolabeled for 10 min with 200 μCi Tran35S-label, and chased at 20°C for 2 h. They were permeabilized according to the swelling technique described by Beckers et al. (28). Permeabilized cells were incubated on ice for 10 min with high salt buffer (20 mM Hepes-KOH, pH 7.2/500 mM potassium acetate/2 mM magnesium acetate/1 mM DTT), and resuspended in transport buffer (20 mM Hepes-KOH, pH 7.2/90 mM potassium acetate/2 mM magnesium acetate/1 mM DTT). The standard incubation mixture contained: permeabilized cells (25–30 μg of protein), 50 μg bovine brain cytosol, 1 mM GTP, and ATP-generating system (1 mM ATP/5 mM creatine phosphate/0.2 units/ml of creatine phosphokinase) in a final volume of 50 μl. In experiments involving treatment with either glycosidases, proteinase K or immunodetection of Golgi integral proteins the assay was scaled up 3–5-fold. Incubation took place at 37°C for 2 h after which the cells were pelleted at 800 × g for 5 min. Unless indicated otherwise both fractions, pellet and supernatant, were solubilized with 1% (vol/vol) Triton X-100 and subjected to immunoprecipitation of VSV-G and SDS/PAGE analysis. Vesicle formation was evaluated by the amount of VSV-G released from cells and expressed as the percentage of the total labeled protein initially present in the cells that appeared in the supernatant. In some experiments, immunoprecipitated VSV-G was digested overnight at 37°C with 2 milliunits of either endoglycosidase H or neuraminidase prior to SDS/PAGE (20).
Electrophoresis and Western Blot Analysis.
Reduced proteins were separated on 7.5% SDS/PAGE. 35S-labeled proteins were quantitated in a PhosphorImager (FUJIX Bas 1000, Tokyo) using the pc-bas, 2.08, program. Protein samples to be analyzed by Western blot analysis were transferred to Immobilon-P (Millipore). Blots were incubated with the appropriate primary antibody followed by horseradish peroxidase-conjugated secondary antibody (Amersham). They were developed using the enhanced chemiluminescence detection system (Amersham) and quantitated by scanning densitometry.
Electron Microscopy.
Cells were cultured on 35-mm dishes and processed as described (20). To estimate the density of coated vesicles in the Golgi area 6–10 consecutive, serial sections from 5–7 different cells were analyzed for each experimental condition. The Golgi area was defined as the cytoplasmic region that contains the Golgi stack and its immediate surroundings including adjacent vesicles and transitional elements of the endoplasmic reticulum, but excluding most of the other organelles such as mitochondria, large endosomes, and endoplasmic reticulum cisternae. For each section a photograph was taken at a final magnification of ×26,700 and the surface of the Golgi area was determined using an electronic pen connected to a microprocessor programmed (videoplan 2.5) to calculate surface area. The volume of each Golgi area was determined by multiplying the mean surface by the thickness of the section (≈70 nm) and the number of individual sections included in the analysis. Free-coated vesicles with a diameter <70 nm were counted on each section.
RESULTS
Inhibitors of PKA Activity Block Transport of VSV-G Glycoprotein from the TGN to the Cell Surface.
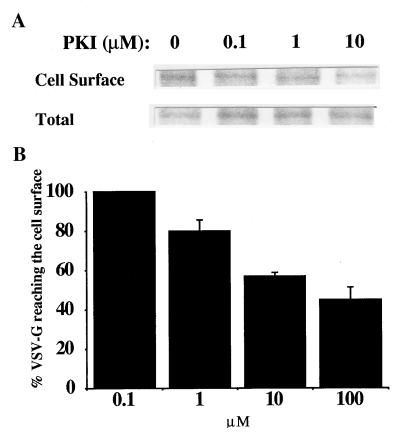
To assess the influence of PKA activity on vesicle-mediated protein transport processes we examined the effects derived from incubating intact cells with selective PKA inhibitors. A lipid derivative of PKI, the inhibitory peptide sequence of the heat-stable PKA inhibitor, was first used. The heat-stable PKA inhibitor is a natural, highly specific inhibitor of C-PKA (29). VSV-infected cells were radiolabeled and incubated at 20°C to accumulate newly synthesized VSV-G in the TGN (30). Transport to the cell surface of this integral membrane glycoprotein was reestablished by shifting the cells to 37°C and analyzed in the presence of increasing concentrations of myristoyl-PKI. Radiolabeled VSV-G was immunoprecipitated from the cell surface and evaluated by SDS/PAGE. Transport was decreased in cells treated with myristoyl-PKI (Fig. 1A). This inhibition was not complete, however; 40–45% of newly synthesized VSV-G molecules still reached the cell surface during 40 min transport. During the same period of time >65% of the VSV-G molecules were detected at the cell surface in control, untreated cells. Maximal inhibition occurred with 10–20 μM myristoyl-PKI; no further inhibition was obtained at higher concentrations (Fig. 1B). In contrast, transport from the TGN to the cell surface was strongly inhibited in the continuous presence of H89 (Fig. 2A, lane 0 of samples treated with H89), a cell permeable inhibitor of C-PKA (31). Used at 35–50 μM this isoquinolinesulfonamide derivative blocked transfer of VSV-G from the TGN to the cell surface. This corroborates previous results with this reagent (20). In addition, it suggests an inefficient inhibition of cellular C-PKA by myristoyl-PKI. Most likely, the latter inhibitor became anchored to the cell membrane and this should have hindered interaction with soluble C-PKA. Nevertheless, transport inhibition observed with myristoyl-PKI clearly established the involvement of PKA activity in vesicle traffic between the TGN and the plasma membrane. These data extend previous studies on the effects of PKA activators on the same transport route (7, 9, 20).
Figure 1.
Effect of myristoyl-PKI on transport of VSV-G from the TGN to the cell surface. VSV-infected, normal rat kidney cells were radiolabeled with Tran35S-label at 37°C for 5 min and chased at 20°C for 2 h to accumulate newly synthesized VSV-G in the TGN. They were either incubated on ice or, alternatively, exposed to myristoyl-PKI at 20°C for 45 min. Cells were transferred to 37°C and additionally incubated at this temperature for 40 min. (A) VSV-G was immunoprecipitated from the cell surface and analyzed by SDS/PAGE. Total VSV-G present within the cells is shown. (B) The amount of VSV-G reaching the cell surface was calculated. Values were normalized for the total amount of VSV-G glycoprotein present in the cells and expressed as percentage of VSV-G transported in untreated, control cells. Data (mean ± SD) are average of three different experiments.
Figure 2.
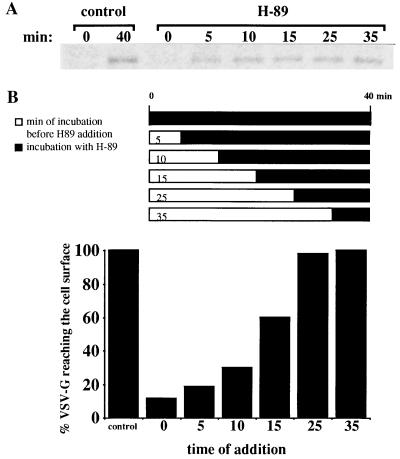
Time course of H89 inhibition of transport of VSV-G from the TGN to the cell surface. Infected, radiolabeled cells were incubated at 20°C for 2 h. They were then transferred to 37°C to allow radiolabeled VSV-G retained in the TGN to be transported to the cell surface. Transport was arrested at the indicated time points; cells were transferred on ice and incubated or not (control) with 50 μM of H89 for 30 min. In the case of H89-treated cells, transport was reestablished by returning the cells to 37°C to complete a 40 min transport period in the continuous presence of this agent. VSV-G was immunoprecipitated from the cell surface and subjected to SDS/PAGE analysis. (A) Access to the cell surface of VSV-G is compared in untreated, control cells and cells incubated with H89. (B) The amount of VSV-G reaching the cell surface is expressed as percentage of that in control cells. Values were normalized for the total amount of VSV-G within the cells. They are representative of three independent experiments.
H89 Inhibits Vesicle Budding from the TGN in Vivo.
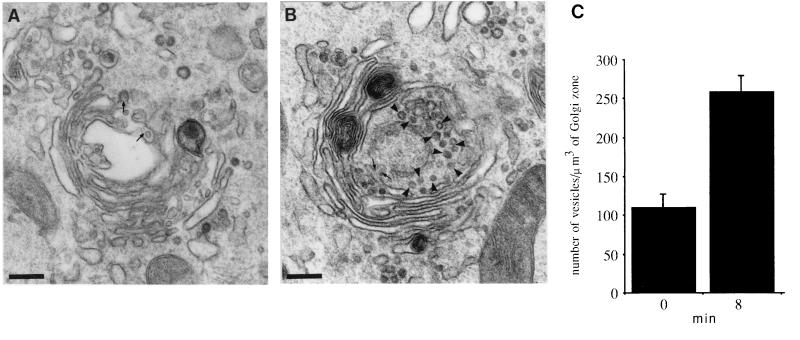
To determine the site of action of PKA in the transport process we analyzed the time at which H89 was effective as inhibitor. Cells containing radiolabeled VSV-G accumulated in the TGN at 20°C were shifted to 37°C and H89 was added at different time points during the transfer of this glycoprotein to the cell surface (Fig. 2). Effective transport inhibition was obtained when H89 was added during the first 5–10 min of transport (Fig. 2B). Addition of H89 at later time points did not significantly affected VSV-G access to the cell surface. This suggested a primary role for PKA in protein export from the TGN rather than vesicle targeting and fusion with the plasma membrane. To support this view we carried out a morphological experiment designed to examine structural changes in the Golgi area during reversal from a H89 block. Cells were maintained at 20°C for 2 h and exposed to H89 during the last 30 min of incubation at this temperature. They were then additionally incubated at 37°C in the continuous presence of H89 for 30 min. This should allow proteins retained in the TGN by the temperature block to gain access to the cell surface. Finally, H89 was washed out and the cells were processed for electron microscopy at different time points during reversal. The Golgi region showed an increase in the number of free-coated vesicles following H89 removal (Fig. 3). While coated buds and vesicles were observed in the presence of H89 (Fig. 3A) the latter increased significantly after incubation in H89-free medium for 5–8 min (Fig. 3B). Apparently, as much as twice vesicles were detected under these conditions (Fig. 3C). They were identified as transport vesicles by immunogold detection of VSV-G (not shown). At later time points the number of free vesicles in the Golgi area decreased as they probably reached the plasma membrane (not shown). Taken together these data indicated that PKA activity was necessary at an early stage of vesicle production in the constitutive transport of VSV-G from the TGN to the cell surface.
Figure 3.
Ultrastructural changes in the Golgi region during reversal from a H89 block. Cells were incubated at 20°C for 2 h. 50 μM H89 was added during the last 30 min of incubation at this temperature. They were transferred to 37°C and additionally incubated with H89 for 30 min. (A) Cells were directly fixed and processed for electron microscopy. (B) Alternatively, they were additionally incubated in H89-free medium for 8 min prior to fixation. Vesicles and buds located at the trans Golgi face are indicated with arrowheads and arrows, respectively. (C) Number of free coated vesicles in the Golgi area determined as described in Materials and Methods. Data (mean ± SD) are the average of at least five different Golgi areas. (Bars = 0.25 μm.)
Effects of PKA Activity on Vesicle Release from the TGN.
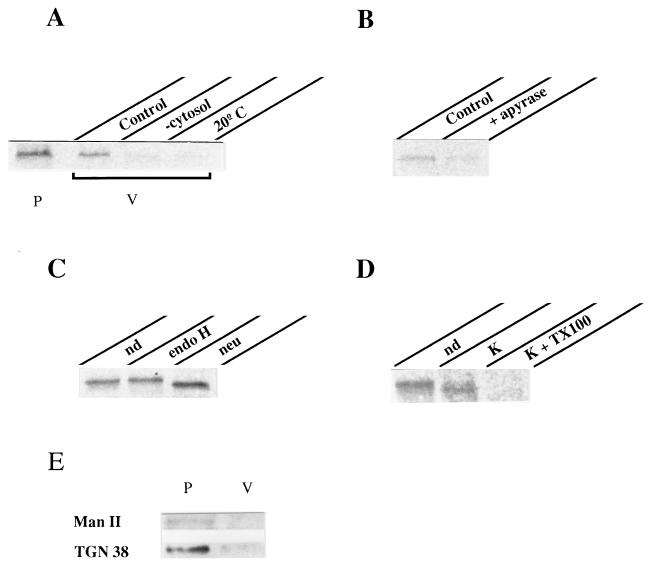
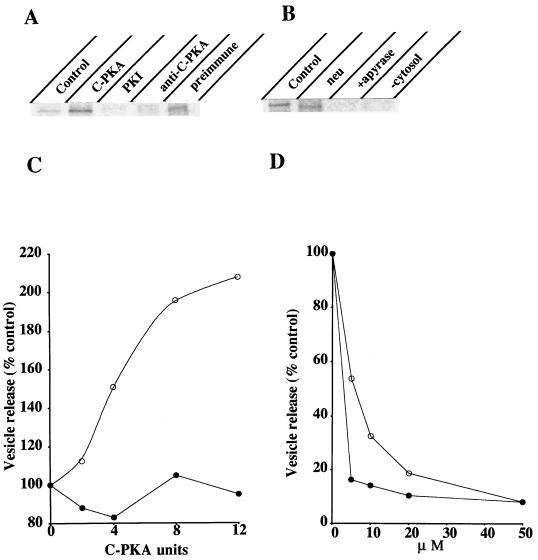
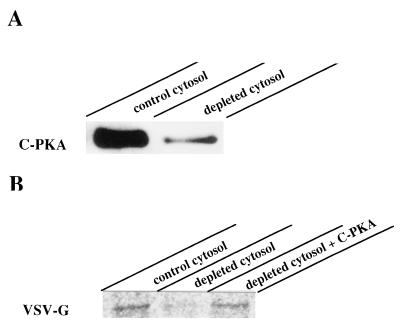
To demonstrate the involvement of PKA activity on vesicle formation we used an in vitro assay previously described to reproduce this transport stage (27). Infected, radiolabeled cells were incubated at 20°C to accumulate VSV-G at the TGN. They were osmotically permeabilized (28), depleted from endogenous cytosolic components, and incubated at 37°C in the presence of exogenous cytosol, GTP, and ATP-generating system. Released vesicles were separated from permeabilized cells by low speed centrifugation, lysed, and subjected to SDS/PAGE analysis after VSV-G immunoprecipitation (Fig. 4). Vesicle budding in this assay accounted for the release of ≈20–30% of the total radiolabeled VSV-G initially present within the cells. It occurred in a temperature and cytosol-dependent manner since almost no VSV-G was released from permeabilized cells maintained at either 4°C or 20°C or incubated in the absence of exogenous cytosol (Fig. 4A). Importantly, vesicle budding required ATP hydrolysis. Addition of apyrase to the assay inhibited VSV-G release and this could not be explained by lack of GTP since apyrase inhibition was also effective in the presence of the nonhydrolyzable GTP analog, GTPγS (Fig. 4B). Released VSV-G was terminally glycosylated as indicated by resistance to endoglycosidase H and sensitivity to neuraminidase digestion (Fig. 4C). In addition, experiments with proteinase K revealed that released VSV-G was contained in intact vesicles (Fig. 4D). Treatment of the vesicle fraction with this protease did not render complete degradation of VSV-G unless Triton X-100 was included. Instead, released VSV-G exposed to proteinase K in the absence of detergent underwent a small increase in its electrophoretic mobility that was probably the result of partial proteolysis affecting its cytoplasmic tail (Fig. 4D). Taken together these data indicated that released VSV-G molecules were confined into sealed vesicles that were for the most part TGN-derived. Integral Golgi proteins such as α-1,2-mannosidase II (25) and TGN38 (24) remained concentrated in the cell pellet after vesicle release (Fig. 4E). In the case of TGN38 a small amount (<5%) was released consistent with the recycling of this protein between the TGN and the plasma membrane (32). These data exclude the possibility of general Golgi vesiculation during the in vitro incubation. We therefore used this assay to evaluate the influence of PKA activity on vesicle formation. As shown in Fig. 5 addition of pure C-PKA subunits to the incubation medium stimulated vesicle release by 2-fold. At least 35% of total VSV-G present within the cells was released into vesicles under C-PKA stimulation. Such stimulation was both cytosol and ATP-dependent and the extra amount of VSV-G released in the presence of C-PKA was sensitive to neuraminidase digestion (Fig. 5B). This indicated that exogenous C-PKA indeed stimulated vesicle budding from the TGN. Functional C-PKA subunits were required because no stimulation occurred when heat-inactivated C-PKA were used (Fig. 5C). Moreover, addition of PKI to the standard assay inhibited vesicle formation (Fig. 5A). This synthetic peptide drastically decreased vesicle release. Whereas >50% inhibition was observed at low concentrations such as 5–10 μM, maximal inhibition (80–90%) required 20–30 μM (Fig. 5D). Addition to the assay of an unrelated peptide only marginally affected vesicle release (<20% inhibition at 35–50 μM). Vesicle production was also inhibited by H89 (Fig. 5D). In this case, 85% inhibition occurred at 5–10 μM, that is 7–10-fold lower than the concentration of this agent needed to inhibit transport in vivo. Vesicle generation from the TGN was abolished by addition to the incubation medium of a specific antibody against C-PKA whereas no effect was observed with preimmune IgG (Fig. 5A). Finally, we examined the effect of using a cytosol preparation with low PKA activity. More than 70% of C-PKA protein was extracted following incubation of native cytosol with cAMP-agarose (Fig. 6A). Vesicle generation was 45% decreased in incubations with this depleted cytosol. After addition of C-PKA the ability of this cytosol preparation to support vesicle budding was restored to 95% of that in control conditions (Fig. 6B). Taken together these results indicated that formation of constitutive transport vesicles at the TGN is modulated by PKA activity.
Figure 4.
Release of post-Golgi vesicles from permeabilized cells. The standard assay consisted of permeabilized cells containing radiolabeled VSV-G accumulated in the TGN, exogenous cytosol, GTP, and ATP-generating system (see Materials and Methods). Incubation took place at 37°C for 2 h. The vesicle fraction (V) was separated from the permeabilized cells (P) by centrifugation and its content in VSV-G determined by immunoprecipitation and SDS/PAGE analysis. (A) Comparison of the amount of VSV-G released into vesicles (V) vs. that remaining within the cells (P) in control conditions. VSV-G released in incubations lacking exogenous cytosol (−cytosol) or performed at low temperature (20°C) is shown. (B) ATP requirement: incubation was carried out in the presence of 1 mM GTPγS instead of GTP and additionally with or without (control) 0.6 units/ml apyrase. (C) Glycosylation of released VSV-G: VSV-G immunoprecipitated from vesicles was subjected or not (nondigested, nd) to either endoglycosidase H (endo H) or neuraminidase (neu) digestion before SDS/PAGE. (D) Vesicle integrity: released vesicles were incubated or not (nd) on ice for 30 min with 0.5 mg/ml proteinase K (K) in the presence or absence of 1% (vol/vol) Triton X-100 (TX100). VSV-G was then immunoprecipitated with 8G5F11 antibody against its extracytoplasmic domain. (E) Detection of both α-1,2-mannosidase II (Man II) and TGN38: 30 μg of membrane proteins from both fractions (P, V) were resolved by SDS/PAGE and determined by Western blot analysis.
Figure 5.
Effects of PKA modulators on vesicle release from the TGN. (A) The standard assay described in Fig. 4 was supplemented or not (control) with either 8 units C-PKA, 20 μM PKI, 2 μg anti-C-PKA antibody or 2 μg preimmune rabbit IgG. (B) Incubation was performed in the presence of 8 units C-PKA but in the absence of either ATP (+apyrase) or cytosol (−cytosol) or in complete medium (control). VSV-G was immunoprecipitated from released, lysed vesicles, and where indicated (neu) subjected to neuraminidase digestion prior to SDS/PAGE analysis. (C) Effect of adding different amounts of both native (○) and heat-inactivated (90°C, 30 min) (•) C-PKA subunits on vesicle release. (D) Effect of adding different amounts of either PKI (○) or H89 (•) on vesicle release. Data are the average of three independent experiments.
Figure 6.
Effects of C-PKA depletion from cytosol on vesicle release. (A) 45 μg of cytosolic proteins from both native bovine brain cytosol (control) and cytosol incubated with cAMP-agarose (depleted) were processed by SDS/PAGE and their content in C-PKA determined by Western blot analysis. (B) The vesicle release assay was performed in the presence of either native cytosol (control), C-PKA-depleted cytosol (depleted), or C-PKA-depleted cytosol supplemented with 8 units C-PKA. VSV-G immunoprecipitated from released, lysed vesicles is shown.
DISCUSSION
In a previous report we have described that transport of newly synthesized VSV-G along the secretory pathway was arrested in cells treated with H89, a specific PKA inhibitor (20). Whereas both endoplasmic reticulum-to-Golgi and intra-Golgi transport steps were sensitive to H89 treatment this agent mostly blocked export out of the Golgi complex to the cell surface. These results contrast with data published by Simon et al. on the production of post-Golgi vesicles from purified Golgi fractions (10). In this study vesicle release was only 50–60% inhibited by H89 while it was sensitive to PKC inhibitors and activators. Moreover, a novel PKC isoform, named PKCμ, has been recently described to be located in the Golgi complex and inhibited by H89 (33). It was, therefore, important that in the present study we establish the involvement of PKA activity in transport from the TGN to the cell surface. The results obtained with myristoyl-PKI indicate that this is indeed the case. This synthetic peptide contains the inhibitory sequence of the thermostable PKA inhibitor, a protein that specifically inhibits PKA activity and has no effect on other kinase families (29). Although transport of VSV-G from the TGN to the cell surface was not completely blocked in cells incubated with myristoyl-PKI it was significantly decreased (Fig. 1). It is reasonable to assume that the inhibitory efficacy of this reagent was limited by its access to the cell interior. It has also been shown that PKI peptide is 10-fold less efficacious than the full-length inhibitor (29). Additionally, inhibition of other kinases in addition to PKA by H89 cannot be excluded at present. Nevertheless, a regulatory role for PKA activity in the TGN-to-cell surface transport is supported by data obtained with activators such as 3-isobutyl-1-methylxanthine and forskolin (7, 9, 20).
Both biochemical (Fig. 2) and morphological (Fig. 3) data obtained in cells treated with H89 indicate that this inhibitor prevents the formation of constitutive transport vesicles from the TGN. In control, untreated cells transfer of VSV-G to the cell surface from the TGN occurs after a 10 min lag (20). This time period seems to be required for coat assembly and vesicle formation. Transport inhibition by H89 occurred during this stage suggesting that PKA activity is necessary to generate the vesicular carriers that mediate transport from the Golgi to the cell surface. In contrast, a role for PKA activity on vesicle targeting and/or fusion seems to be excluded from the absence of H89 inhibitory effect at later time points. Removal of H89 block gave rise to a transient increase in the number of free coated vesicles in the Golgi area. While most of these vesicular profiles were seen associated to TGN elements some of them were located close to the endoplasmic reticulum and proximal Golgi cisternae. It is thus possible that PKA activity affects vesicle formation at more than one location in the secretory pathway.
By using an in vitro assay that reproduces vesicle budding from the TGN (Fig. 4) we have been able to directly test the influence of PKA activity on this process. Vesicle production was stimulated by exogenous C-PKA (Fig. 5) while it was inhibited by depletion of endogenous C-PKA (Fig. 6). Interestingly, the stimulatory effect caused by C-PKA on vesicle budding in vitro roughly matches the increase in the number of free vesicles observed in vivo during H89 reversal. Therefore, data obtained from both in vivo and in vitro systems correlate well and indicate that PKA activity is necessary to generate transport vesicles from the TGN. The inhibitory efficacy of both H89 and PKI was higher in the in vitro assay than in vivo. However, the concentrations of both inhibitors needed to inhibit vesicle release were still far above their Ki for PKA (≈0.08 nM for PKI and 50 nM for H89) (29, 31). In the case of H89 high concentrations (10–50 μM) of this reagent were also used in previous studies to inhibit transport activities (10, 17, 34). It is therefore possible that the interaction in situ of both inhibitors with C-PKA depends on factors such as the particular isoenzyme involved or membrane localization which are unclear at present.
Vesicle budding depends on the assembly of a protein coat that is recruited on the cytosolic side of donor membrane from soluble components (2, 35). The coat is thought to act as a mechanical device that drives membrane deformation into a bud. Several coat proteins (36–39) and proteins that activate coat assembly (40–42) contain PKA phosphorylation sequences. It is therefore possible that PKA activity determines vesicle budding by regulating the interaction of particular sets of coat proteins with intracellular membranes. In addition, or alternatively, PKA could have a role in cargo selection and packaging at the TGN.
Acknowledgments
We thank Drs. Enrique Rodriguez Boulan and Anne Muesch (Cornell University) for helpful suggestions relative to the assay for vesicle production from the TGN and Drs. D. S. Lyles, G. Banting, and M. G. Farquhar for generous gifts of antibodies. This work was funded by Grant 97/1170 from the Spanish Fondo de Investigaciones Sanitarias.
ABBREVIATIONS
- PKA
protein kinase A
- C-PKA
PKA catalytic subunit
- PKI
PKA inhibitory peptide
- PKC
protein kinase C
- TGN
trans-Golgi network
- H89
N-[2-(-p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide
- VSV-G
vesicular stomatitis virus G glycoprotein
References
- 1.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 2.Schekman R, Orci L. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 3.Stack J H, Herman P K, Schu P V, Emr S D. EMBO J. 1993;12:2195–2204. doi: 10.1002/j.1460-2075.1993.tb05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown W J, DeWald D B, Emr S D, Plutner H, Balch W E. J Cell Biol. 1995;130:781–796. doi: 10.1083/jcb.130.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson H W. J Cell Biol. 1995;130:797–805. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin C D, Shields D. J Cell Biol. 1996;135:1471–1483. doi: 10.1083/jcb.135.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pimplikar S W, Simons K. J Biol Chem. 1994;269:19054–19059. [PubMed] [Google Scholar]
- 8.Buccione R, Bannykh S, Santone I, Baldassarre M, Facchiano F, Bozzi Y, Tullio G D, Mironov A, Luini A, Matteis M A D. J Biol Chem. 1996;271:3523–3533. doi: 10.1074/jbc.271.7.3523. [DOI] [PubMed] [Google Scholar]
- 9.Jilling T, Kirk K L. J Biol Chem. 1996;271:4381–4387. doi: 10.1074/jbc.271.8.4381. [DOI] [PubMed] [Google Scholar]
- 10.Simon J-P, Ivanov I E, Shopsin B, Hersh D, Adesnik M, Sabatini D D. J Biol Chem. 1996;271:16952–16961. doi: 10.1074/jbc.271.28.16952. [DOI] [PubMed] [Google Scholar]
- 11.Westermann P, Knoblich M, Maier O, Lindschau C, Haller H. Biochem J. 1996;320:651–658. doi: 10.1042/bj3200651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozawa K, Szallasi Z, Kazanietz M G, Blumberg P M, Mischak H, Mushinski J F, Beaven M A. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 13.Akita Y, Ohno S, Yajima Y, Konno Y, Saido T C, Mizuno K, Chida K, Osada S, Kuroki T, Kawashima S, Suzuki K. J Biol Chem. 1994;269:4653–4660. [PubMed] [Google Scholar]
- 14.Takuma T, Ichida T. J Biol Chem. 1994;269:22124–22128. [PubMed] [Google Scholar]
- 15.Xu H, Greengard P, Gandy S. J Biol Chem. 1995;270:23243–23245. doi: 10.1074/jbc.270.40.23243. [DOI] [PubMed] [Google Scholar]
- 16.Cardone M H, Smith B L, Song W, Mochly-Rosen D, Mostov K E. J Cell Biol. 1994;124:717–727. doi: 10.1083/jcb.124.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen S H, Casanova J E. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Matteis M A, Santini G, Kahn R A, Di Tullio G, Luini A. Science. 1993;364:818–820. doi: 10.1038/364818a0. [DOI] [PubMed] [Google Scholar]
- 19.Simon J-P, Ivanov I E, Adesnik M, Sabatini D D. J Cell Biol. 1996;135:355–370. doi: 10.1083/jcb.135.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñiz M, Alonso M, Hidalgo J, Velasco A. J Biol Chem. 1996;271:30935–30941. doi: 10.1074/jbc.271.48.30935. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra V, Serafini T, Orci L, Shepherd J C, Rothman J E. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 22.Le François L, Lyles D S. Virology. 1982;121:157–167. [Google Scholar]
- 23.Kreis T E. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzio J P, Brake B, Banting G, Howell K E, Braghetta P, Stanley K K. Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velasco A, Hendricks L, Moremen K W, Tulsiani D R P, Touster O, Farquhar M G. J Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson H W, McGowan C H, Balch W E. J Cell Biol. 1992;116:1343–1355. doi: 10.1083/jcb.116.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müsch A, Xu H, Shields D, Rodriguez-Boulan E. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckers C J M, Keller D S, Balch W E. Cell. 1987;50:523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- 29.Walsh D A, Angelos K L, Patten S M V, Glass D B, Garetto L P. In: Peptides and Protein Phosphorylation. Kemp B E, editor. Boca Raton, FL: CRC; 1990. pp. 43–84. [Google Scholar]
- 30.Griffiths G, Pfeiffer S, Simons K, Matlin K. J Cell Biol. 1985;101:949–963. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 32.Bos K, Wraight C, Stanley K K. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prestle J, Pfizenmaier K, Brenner J, Johannes F-J. J Cell Biol. 1996;134:1401–1410. doi: 10.1083/jcb.134.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zegers M M P, Hoekstra D. J Cell Biol. 1997;138:307–321. doi: 10.1083/jcb.138.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bednareck S Y, Orci L, Schekman R. Trends Cell Biol. 1996;6:468–473. doi: 10.1016/0962-8924(96)84943-9. [DOI] [PubMed] [Google Scholar]
- 36.Sheff D, Lowe M, Kreis T E, Mellman I. J Biol Chem. 1996;271:7230–7236. doi: 10.1074/jbc.271.12.7230. [DOI] [PubMed] [Google Scholar]
- 37.Wilde A, Brodsky F M. J Cell Biol. 1996;135:635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dell’Angelica E C, Ooi C E, Bonifacino J S. J Biol Chem. 1997;272:15078–15084. doi: 10.1074/jbc.272.24.15078. [DOI] [PubMed] [Google Scholar]
- 39.Salama N R, Chuang J S, Schekman R W. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chardin P, Paris S, Antonny B, Robineau S, Béraud-Dufour S, Jackson C L, Chabre M. Nature (London) 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 41.Peryoche A, Paris S, Jackson C L. Nature (London) 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- 42.Rosa J L, Casaroli-Marano R P, Buckler A J, Vilaró S, Barbacid M. EMBO J. 1996;15:4262–4273. [PMC free article] [PubMed] [Google Scholar]