Abstract
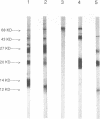
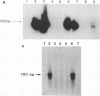
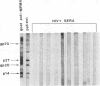
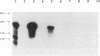
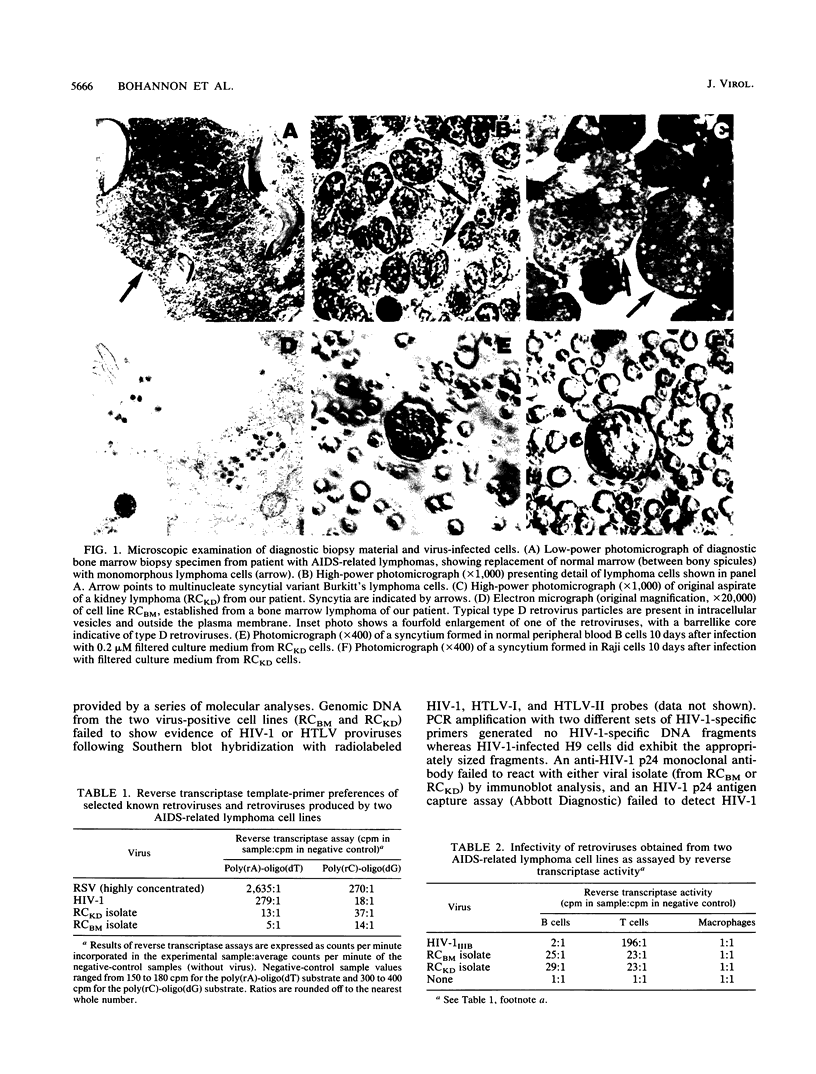
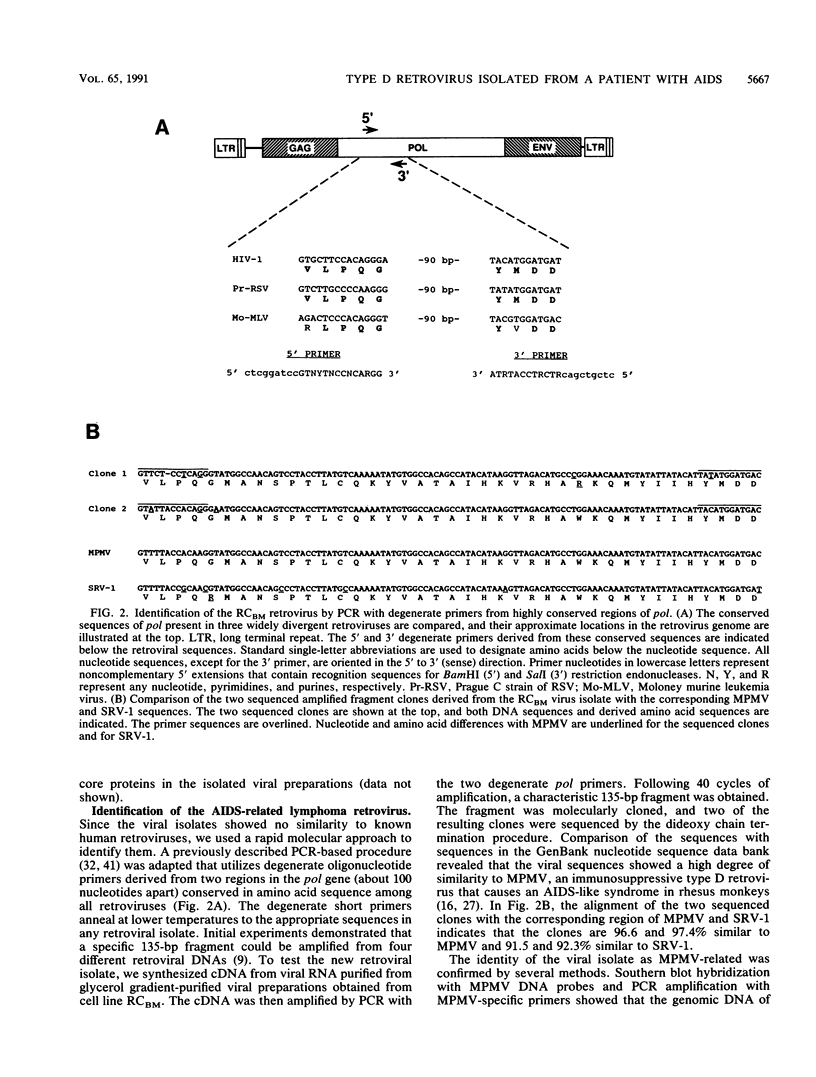
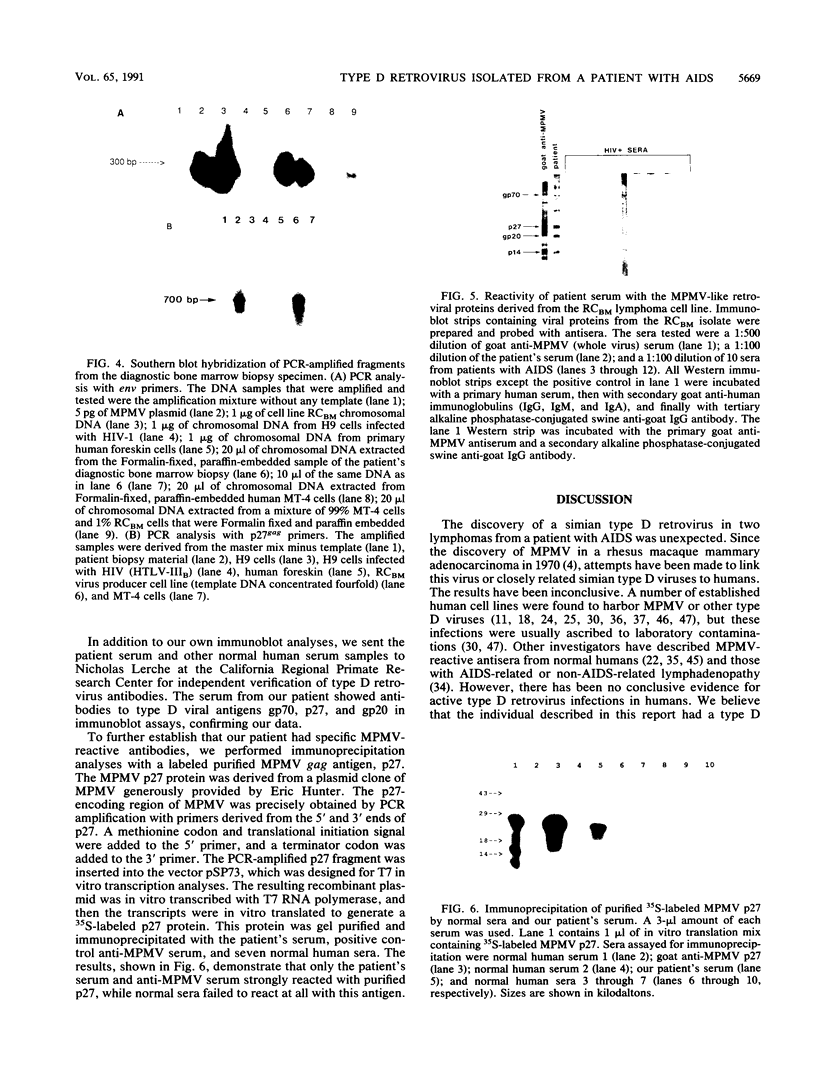
An atypical syncytial variant of a high-grade Burkitt's-type B-cell lymphoma from a patient with AIDS who was seropositive for human immunodeficiency virus type 1 was studied. A productive type D retrovirus infection was identified in early-passage cell lines derived from two lymphomas from this patient. Nucleotide and amino acid sequence analysis as well as immunologic reactivity indicated that the isolated virus was highly related to Mason-Pfizer monkey virus (MPMV). MPMV is an immunosuppressive type D retrovirus that causes an AIDS-like syndrome in rhesus macaques. Amplification of DNA from the patient's diagnostic bone marrow biopsy specimen by polymerase chain reaction generated the appropriate MPMV-specific fragments and indicated that the patient was infected with the MPMV-like retrovirus. In addition, the patient's serum contained antibodies which recognized type D viral env proteins (gp70 and gp20) and gag proteins (p27 and p14). Although there have been reports of human cell lines infected with type D retroviruses and of type D-reactive human sera, this is the first evidence of a type D retrovirus infection in a human confirmed by virus isolation, serum reactivity, and viral DNA identification in tumor tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. L., Gardner M. B., Marx P. A., Maul D. H., Lerche N. W., Osborn K. G., Lowenstine L. J., Bodgen A., Arthur L. O., Hunter E. Immunodeficiency in rhesus monkeys associated with the original Mason-Pfizer monkey virus. J Natl Cancer Inst. 1986 Oct;77(4):957–965. [PubMed] [Google Scholar]
- Charman H. P., Rahman R., White M. H., Kim N., Gilden R. V. Radioimmunoassay for the major structural protein of Mason-Pfizer monkey virus: Attempts to detect the presence of antigen or antibody in humans. Int J Cancer. 1977 Apr 15;19(4):498–504. doi: 10.1002/ijc.2910190410. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Daniel M. D., King N. W., Letvin N. L., Hunt R. D., Sehgal P. K., Desrosiers R. C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984 Feb 10;223(4636):602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Schmidt D. K., Silva D. P., Solomon K. R., Hodi F. S., Jr, Ringler D. J., Hunt R. D., King N. W. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer. 1988 Apr 15;41(4):601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Bohannon R. C., Ford R. J., Gibbs R. A. The use of primers from highly conserved pol regions to identify uncharacterized retroviruses by the polymerase chain reaction. J Virol Methods. 1990 Apr;28(1):33–46. doi: 10.1016/0166-0934(90)90085-t. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Arthur L. O. Expression of natural antibodies against endogenous and horizontally transmitted macaque retroviruses in captive primates. Virology. 1981 Jul 15;112(1):49–61. doi: 10.1016/0042-6822(81)90611-5. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Devare S. G., Arthur L. O., Charman H. P., Stephenson J. R. Type D retroviruses: occurrence of natural antibodies to Mason-Pfizer monkey virus in rhesus monkeys. Virology. 1978 May 15;86(2):567–571. doi: 10.1016/0042-6822(78)90096-x. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Landon J. C., Pienta R. J., Kubicek M. T., Valerio M. G., Loeb W. F., Chopra H. C. Responses of infant rhesus monkeys to inoculation with Mason-Pfizer monkey virus materials. J Natl Cancer Inst. 1975 Mar;54(3):651–658. [PubMed] [Google Scholar]
- Fine D., Schochetman G. Type D primate retroviruses: a review. Cancer Res. 1978 Oct;38(10):3123–3139. [PubMed] [Google Scholar]
- Ford R. J., Goodacre A., Ramirez I., Mehta S. R., Cabanillas F. Establishment and characterization of human B-cell lymphoma cell lines using B-cell growth factor. Blood. 1990 Mar 15;75(6):1311–1318. [PubMed] [Google Scholar]
- Gardner M. B., Luciw P., Lerche N., Marx P. Nonhuman primate retrovirus isolates and AIDS. Adv Vet Sci Comp Med. 1988;32:171–226. doi: 10.1016/b978-0-12-039232-2.50011-6. [DOI] [PubMed] [Google Scholar]
- Gelderblom H., Bauer H., Ogura H., Wigand R., Fischer A. B. Detection of oncornavirus-like particles in HeLa cells. I. Fine structure and comparative morphological classification. Int J Cancer. 1974 Feb 15;13(2):246–253. doi: 10.1002/ijc.2910130212. [DOI] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Flügel R. M., Walder R. Retroviral antibodies in Indians. Nature. 1990 May 10;345(6271):120–120. doi: 10.1038/345120a0. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Blake B. J., Chalifoux L. V., Sehgal P. K., King N. W., Letvin N. L. Transmission of naturally occurring lymphoma in macaque monkeys. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5085–5089. doi: 10.1073/pnas.80.16.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin K. V., Bykovsky A. F., Zhdanov V. M. An oncornavirus isolated from human cancer cell line. Cancer. 1973 Jul;32(1):89–96. doi: 10.1002/1097-0142(197307)32:1<89::aid-cncr2820320112>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Alroy J., Essex M. Isolation of T-lymphotropic retrovirus related to HTLV-III/LAV from wild-caught African green monkeys. Science. 1985 Nov 22;230(4728):951–954. doi: 10.1126/science.2997923. [DOI] [PubMed] [Google Scholar]
- King N. W. Simian models of acquired immunodeficiency syndrome (AIDS): a review. Vet Pathol. 1986 Jul;23(4):345–353. doi: 10.1177/030098588602300401. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause H., Wunderlich V., Uckert W. Molecular cloning of a type D retrovirus from human cells (PMFV) and its homology to simian acquired immunodeficiency type D retroviruses. Virology. 1989 Nov;173(1):214–222. doi: 10.1016/0042-6822(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Eaton K. A., Aldrich W. R., Sehgal P. K., Blake B. J., Schlossman S. F., King N. W., Hunt R. D. Acquired immunodeficiency syndrome in a colony of macaque monkeys. Proc Natl Acad Sci U S A. 1983 May;80(9):2718–2722. doi: 10.1073/pnas.80.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack D. H., Sninsky J. J. A sensitive method for the identification of uncharacterized viruses related to known virus groups: hepadnavirus model system. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6977–6981. doi: 10.1073/pnas.85.18.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P. A., Maul D. H., Osborn K. G., Lerche N. W., Moody P., Lowenstine L. J., Henrickson R. V., Arthur L. O., Gilden R. V., Gravell M. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984 Mar 9;223(4640):1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- Morozov V. A., Ilyinskii P. O., Uckert W. A., Wunderlich W., Ilyin K. V. Antibodies to structural and nonstructural gag-coded proteins of type-D retroviruses in humans with lymphadenopathy and AIDS. Int J Tissue React. 1989;11(1):1–5. [PubMed] [Google Scholar]
- Morozov V. A., Saal F., Gessain A., Terrinha A., Périès J. Antibodies to gag gene coded polypeptides of type D retroviruses in healthy people from Guinea-Bissau. Intervirology. 1991;32(4):253–257. doi: 10.1159/000150207. [DOI] [PubMed] [Google Scholar]
- Oda T., Ikeda S., Watanabe S., Hatsushika M., Akiyama K., Mitsunobu F. Molecular cloning, complete nucleotide sequence, and gene structure of the provirus genome of a retrovirus produced in a human lymphoblastoid cell line. Virology. 1988 Dec;167(2):468–476. [PubMed] [Google Scholar]
- Popovic M., Kalyanaraman V. S., Reitz M. S., Sarngadharan M. G. Identification of the RPMI 8226 retrovirus and its dissemination as a significant contaminant of some widely used human and marmoset cell lines. Int J Cancer. 1982 Jul 15;30(1):93–99. doi: 10.1002/ijc.2910300116. [DOI] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selik R. M., Starcher E. T., Curran J. W. Opportunistic diseases reported in AIDS patients: frequencies, associations, and trends. AIDS. 1987 Sep;1(3):175–182. [PubMed] [Google Scholar]
- Shih A., Misra R., Rush M. G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989 Jan;63(1):64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Bossens M., Cogniaux-Le Clerc J., Vereerstraeten P. Neutralization of Mason-Pfizer virus by sera from patients treated for renal disease. J Gen Virol. 1978 Dec;41(3):587–597. doi: 10.1099/0022-1317-41-3-587. [DOI] [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Bossens M., Neuray F. Cell-mediated immune response to simian oncornavirus antigens in pregnant women. J Natl Cancer Inst. 1978 Mar;60(3):527–532. doi: 10.1093/jnci/60.3.527. [DOI] [PubMed] [Google Scholar]