Abstract
The S2 gene nucleotide sequences of prototype strains of the three reovirus serotypes were determined to gain insight into the structure and function of the S2 translation product, virion core protein sigma 2. The S2 sequences of the type 1 Lang, type 2 Jones, and type 3 Dearing strains are 1,331 nucleotides in length and contain a single large open reading frame that could encode a protein of 418 amino acids, corresponding to sigma 2. The deduced sigma 2 amino acid sequences of these strains are very conserved, being identical at 94% of the sequence positions. Predictions of sigma 2 secondary structure and hydrophobicity suggest that the protein has a two-domain structure. A larger domain is suggested to be formed from the amino-terminal three-fourths of sigma 2 sequence, which is separated from a smaller carboxy-terminal domain by a turn-rich hinge region. The carboxy-terminal domain includes sequences that are more hydrophilic than those in the rest of the protein and contains sequences which are predicted to form an alpha-helix. A region of striking similarity was found between amino acids 354 and 374 of sigma 2 and amino acids 1008 and 1031 of the beta subunit of the Escherichia coli DNA-dependent RNA polymerase. We suggest that the regions with similar sequence in sigma 2 and the beta subunit form amphipathic alpha-helices which may play a related role in the function of each protein. We have also performed experiments to further characterize the double-stranded RNA-binding activity of sigma 2 and found that the capacity to bind double-stranded RNA is a property of the sigma 2 protein of prototype strains and of the S2 mutant tsC447.
Full text
PDF


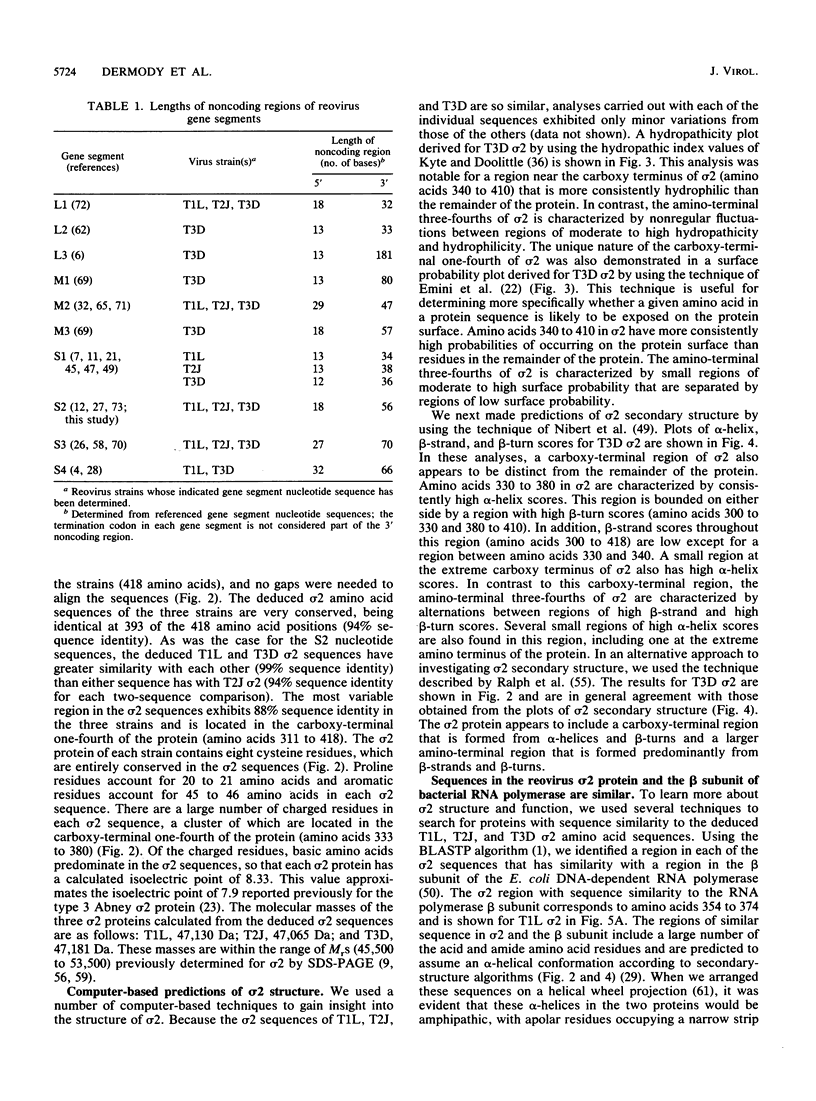





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anders D. G., Consigli R. A. Chemical cleavage of polyomavirus major structural protein VP1: identification of cleavage products and evidence that the receptor moiety resides in the carboxy-terminal region. J Virol. 1983 Oct;48(1):197–205. doi: 10.1128/jvi.48.1.197-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater J. A., Munemitsu S. M., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain s4 mRNA which encodes the major capsid surface polypeptide sigma 3. Biochem Biophys Res Commun. 1986 Apr 14;136(1):183–192. doi: 10.1016/0006-291x(86)90893-4. [DOI] [PubMed] [Google Scholar]
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Bartlett J. A., Joklik W. K. The sequence of the reovirus serotype 3 L3 genome segment which encodes the major core protein lambda 1. Virology. 1988 Nov;167(1):31–37. doi: 10.1016/0042-6822(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R., Jayasuriya A., Chatterjee D., Sonenberg N., Maizel J. V., Jr, Fields B. N. Sequence of reovirus haemagglutinin predicts a coiled-coil structure. 1985 May 30-Jun 5Nature. 315(6018):421–423. doi: 10.1038/315421a0. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R., Spriggs D. R., Tyler K. L., Fields B. N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986 Oct;60(1):64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar L. W., Chmelo R. A., Wiener J. R., Joklik W. K. Sequences of the S1 genes of the three serotypes of reovirus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):24–28. doi: 10.1073/pnas.82.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar L. W., Esparza J., Hudson G. R., Chmelo R., Lee P. W., Joklik W. K. Cloning the double-stranded RNA genes of reovirus: sequence of the cloned S2 gene. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7644–7648. doi: 10.1073/pnas.79.24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. T., Zweerink H. J. Fate of parental reovirus in infected cell. Virology. 1971 Dec;46(3):544–555. doi: 10.1016/0042-6822(71)90058-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cleveland D. R., Zarbl H., Millward S. Reovirus guanylyltransferase is L2 gene product lambda 2. J Virol. 1986 Oct;60(1):307–311. doi: 10.1128/jvi.60.1.307-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K. M., Fields B. N., Harrison S. C. Crystallization of the reovirus type 3 Dearing core. Crystal packing is determined by the lambda 2 protein. J Mol Biol. 1990 Sep 5;215(1):1–5. doi: 10.1016/s0022-2836(05)80089-0. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dermody T. S., Nibert M. L., Bassel-Duby R., Fields B. N. Sequence diversity in S1 genes and S1 translation products of 11 serotype 3 reovirus strains. J Virol. 1990 Oct;64(10):4842–4850. doi: 10.1128/jvi.64.10.4842-4850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Activation and characterization of the reovirus transcriptase: genetic analysis. J Virol. 1982 Jan;41(1):110–118. doi: 10.1128/jvi.41.1.110-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Horne D., Cashdollar L. W., Joklik W. K., Lee P. W. Identification of conserved domains in the cell attachment proteins of the three serotypes of reovirus. Virology. 1990 Feb;174(2):399–409. doi: 10.1016/0042-6822(90)90093-7. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing D. D., Sargent M. D., Borsa J. Switch-on of transcriptase function in reovirus: analysis of polypeptide changes using 2-D gels. Virology. 1985 Jul 30;144(2):448–456. doi: 10.1016/0042-6822(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Fukusho A., Yu Y., Yamaguchi S., Roy P. Completion of the sequence of bluetongue virus serotype 10 by the characterization of a structural protein, VP6, and a non-structural protein, NS2. J Gen Virol. 1989 Jul;70(Pt 7):1677–1689. doi: 10.1099/0022-1317-70-7-1677. [DOI] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C. X., Crowe A., Munemitsu S. M., Atwater J. A., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain s2 mRNA which encodes the virion core polypeptide sigma 2. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1153–1161. doi: 10.1016/s0006-291x(87)80190-0. [DOI] [PubMed] [Google Scholar]
- Giantini M., Seliger L. S., Furuichi Y., Shatkin A. J. Reovirus type 3 genome segment S4: nucleotide sequence of the gene encoding a major virion surface protein. J Virol. 1984 Dec;52(3):984–987. doi: 10.1128/jvi.52.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. E., Jones S. T., Nene V., Nomura T., Fujita N., Ishihama A. Genetic studies on the beta subunit of Escherichia coli RNA polymerase. VIII. Localisation of a region involved in promoter selectivity. Mol Gen Genet. 1986 Jun;203(3):487–491. doi: 10.1007/BF00422074. [DOI] [PubMed] [Google Scholar]
- Grachev M. A., Lukhtanov E. A., Mustaev A. A., Zaychikov E. F., Abdukayumov M. N., Rabinov I. V., Richter V. I., Skoblov Y. S., Chistyakov P. G. Studies of the functional topography of Escherichia coli RNA polymerase. A method for localization of the sites of affinity labelling. Eur J Biochem. 1989 Apr 1;180(3):577–585. doi: 10.1111/j.1432-1033.1989.tb14684.x. [DOI] [PubMed] [Google Scholar]
- Jayasuriya A. K., Nibert M. L., Fields B. N. Complete nucleotide sequence of the M2 gene segment of reovirus type 3 dearing and analysis of its protein product mu 1. Virology. 1988 Apr;163(2):591–602. doi: 10.1016/0042-6822(88)90300-5. [DOI] [PubMed] [Google Scholar]
- Kashlev M., Lee J., Zalenskaya K., Nikiforov V., Goldfarb A. Blocking of the initiation-to-elongation transition by a transdominant RNA polymerase mutation. Science. 1990 May 25;248(4958):1006–1009. doi: 10.1126/science.1693014. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landon Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Leszczynski J. F., Rose G. D. Loops in globular proteins: a novel category of secondary structure. Science. 1986 Nov 14;234(4778):849–855. doi: 10.1126/science.3775366. [DOI] [PubMed] [Google Scholar]
- Mallon R. G., Wojciechowicz D., Defendi V. DNA-binding activity of papillomavirus proteins. J Virol. 1987 May;61(5):1655–1660. doi: 10.1128/jvi.61.5.1655-1660.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. B., Both G. W. Completion of the genomic sequence of the simian rotavirus SA11: nucleotide sequences of segments 1, 2, and 3. Virology. 1990 Jul;177(1):324–331. doi: 10.1016/0042-6822(90)90487-c. [DOI] [PubMed] [Google Scholar]
- Morozov S. Y. A possible relationship of reovirus putative RNA polymerase to polymerases of positive-strand RNA viruses. Nucleic Acids Res. 1989 Jul 11;17(13):5394–5394. doi: 10.1093/nar/17.13.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S. M., Atwater J. A., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain bicistronic s1 mRNA which encodes the minor capsid polypeptide sigma 1a and the nonstructural polypeptide sigma 1bNS. Biochem Biophys Res Commun. 1986 Oct 30;140(2):508–514. doi: 10.1016/0006-291x(86)90761-8. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Nagata L., Masri S. A., Mah D. C., Lee P. W. Molecular cloning and sequencing of the reovirus (serotype 3) S1 gene which encodes the viral cell attachment protein sigma 1. Nucleic Acids Res. 1984 Nov 26;12(22):8699–8710. doi: 10.1093/nar/12.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M. L., Dermody T. S., Fields B. N. Structure of the reovirus cell-attachment protein: a model for the domain organization of sigma 1. J Virol. 1990 Jun;64(6):2976–2989. doi: 10.1128/jvi.64.6.2976-2989.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Ralph S. J., Harvey J. D., Bellamy A. R. Subunit structure of the reovirus spike. J Virol. 1980 Dec;36(3):894–896. doi: 10.1128/jvi.36.3.894-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph W. W., Webster T., Smith T. F. A modified Chou and Fasman protein structure algorithm. Comput Appl Biosci. 1987 Sep;3(3):211–216. doi: 10.1093/bioinformatics/3.3.211. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Mustoe T. A., Sharpe A. H., Fields B. N. A genetic map of reovirus. II. Assignment of the double-stranded RNA-negative mutant groups C, D, and E to genome segments. Virology. 1978 Apr;85(2):531–534. doi: 10.1016/0042-6822(78)90459-2. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Furuichi Y. Nucleotide sequence of reovirus genome segment S3, encoding non-structural protein sigma NS. Nucleic Acids Res. 1983 Sep 24;11(18):6399–6408. doi: 10.1093/nar/11.18.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seliger L. S., Zheng K., Shatkin A. J. Complete nucleotide sequence of reovirus L2 gene and deduced amino acid sequence of viral mRNA guanylyltransferase. J Biol Chem. 1987 Dec 5;262(34):16289–16293. [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Automatic generation of primary sequence patterns from sets of related protein sequences. Proc Natl Acad Sci U S A. 1990 Jan;87(1):118–122. doi: 10.1073/pnas.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow O., McCorquodale J. G., McCrae M. A. Molecular cloning and sequencing of the gene (M2) encoding the major virion structural protein (mu 1-mu 1C) of serotypes 1 and 3 of mammalian reovirus. Virology. 1988 May;164(1):141–146. doi: 10.1016/0042-6822(88)90629-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. K., Zweerink H. J. Studies on the structure of reovirus cores: selective removal of polypeptide lambda 2. Virology. 1976 Mar;70(1):171–180. doi: 10.1016/0042-6822(76)90247-6. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Bartlett J. A., Joklik W. K. The sequences of reovirus serotype 3 genome segments M1 and M3 encoding the minor protein mu 2 and the major nonstructural protein mu NS, respectively. Virology. 1989 Apr;169(2):293–304. doi: 10.1016/0042-6822(89)90154-2. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Joklik W. K. Comparison of the reovirus serotype 1, 2, and 3 S3 genome segments encoding the nonstructural protein sigma NS. Virology. 1987 Dec;161(2):332–339. doi: 10.1016/0042-6822(87)90125-5. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Joklik W. K. Evolution of reovirus genes: a comparison of serotype 1, 2, and 3 M2 genome segments, which encode the major structural capsid protein mu 1C. Virology. 1988 Apr;163(2):603–613. doi: 10.1016/0042-6822(88)90301-7. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Joklik W. K. The sequences of the reovirus serotype 1, 2, and 3 L1 genome segments and analysis of the mode of divergence of the reovirus serotypes. Virology. 1989 Mar;169(1):194–203. doi: 10.1016/0042-6822(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., McLaughlin T., Joklik W. K. The sequences of the S2 genome segments of reovirus serotype 3 and of the dsRNA-negative mutant ts447. Virology. 1989 May;170(1):340–341. doi: 10.1016/0042-6822(89)90392-9. [DOI] [PubMed] [Google Scholar]