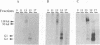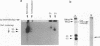Abstract
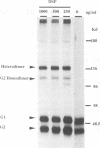
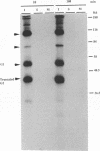
We have investigated the oligomerization and intracellular transport of the membrane glycoproteins of Punta Toro virus, a member of the Phlebovirus genus of the family Bunyaviridae, which is assembled by budding in the Golgi complex. By using one- or two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis, chemical cross-linking, and sucrose gradient centrifugation, we found that the majority of the G1 and G2 glycoproteins are assembled into noncovalently linked G1-G2 heterodimers. At the same time, a fraction of the G2 protein, possibly produced independently of the G1 protein, is assembled into G2 homodimers. Kinetic analysis indicates that heterodimerization occurs between newly synthesized G1 and G2 within 3 min after protein synthesis, and that the G1 and G2 glycoproteins are associated as dimeric forms both during transport and after accumulation in the Golgi complex. Analysis of a G1-truncated G2 mutant, which is also targeted to the Golgi complex, showed that these molecules also assemble into dimeric forms, which are linked by disulfide bonds. Both the G1-G2 heterodimer and the G2 homodimer were found to be able to exit from the endoplasmic reticulum. Differences in transport kinetics observed for the G1 and G2 proteins may be due to the differences in the transport efficiency between the G1-G2 heterodimer and the G2 homodimer from the endoplasmic reticulum to the Golgi complex. These and previous results (S.-Y. Chen, Y. Matsuoka, and R.W. Compans, Virology 183:351-365, 1991) suggest that Golgi retention of the G2 homodimer occurs by association with the G1-G2 heterodimer, whereas the Golgi targeting of the G1-G2 heterodimer occurs by a specific retention mechanism.
Full text
PDF


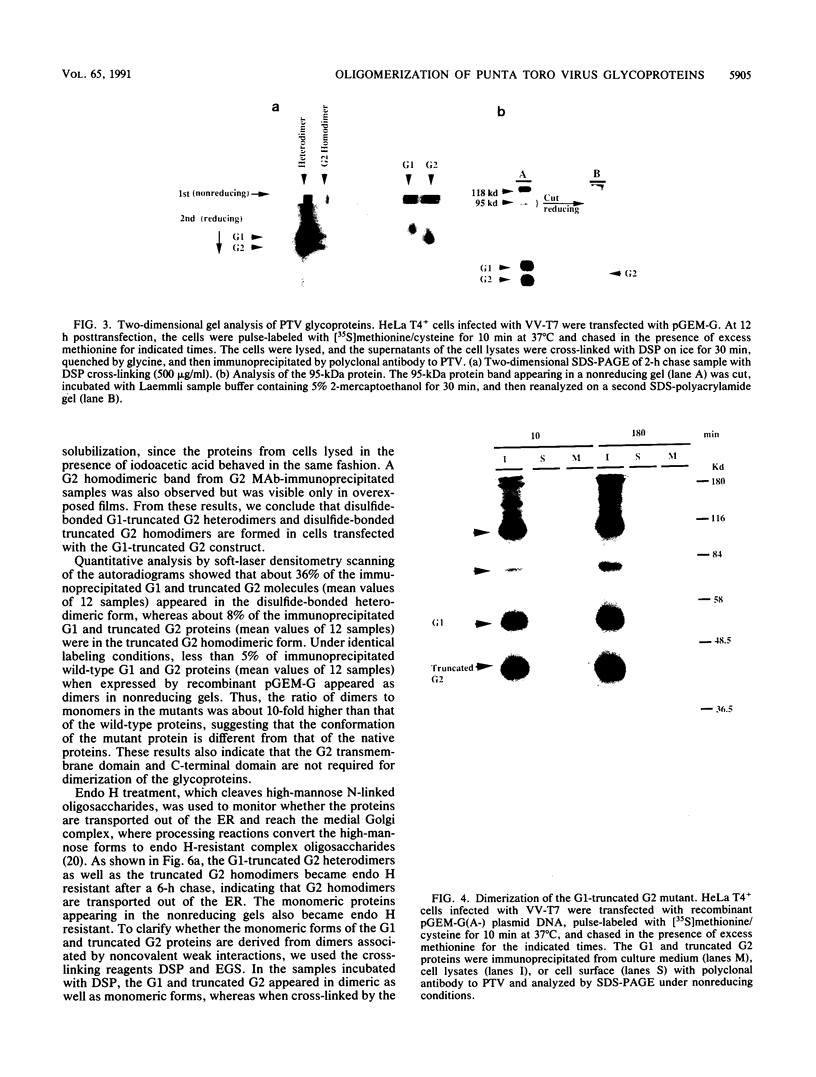
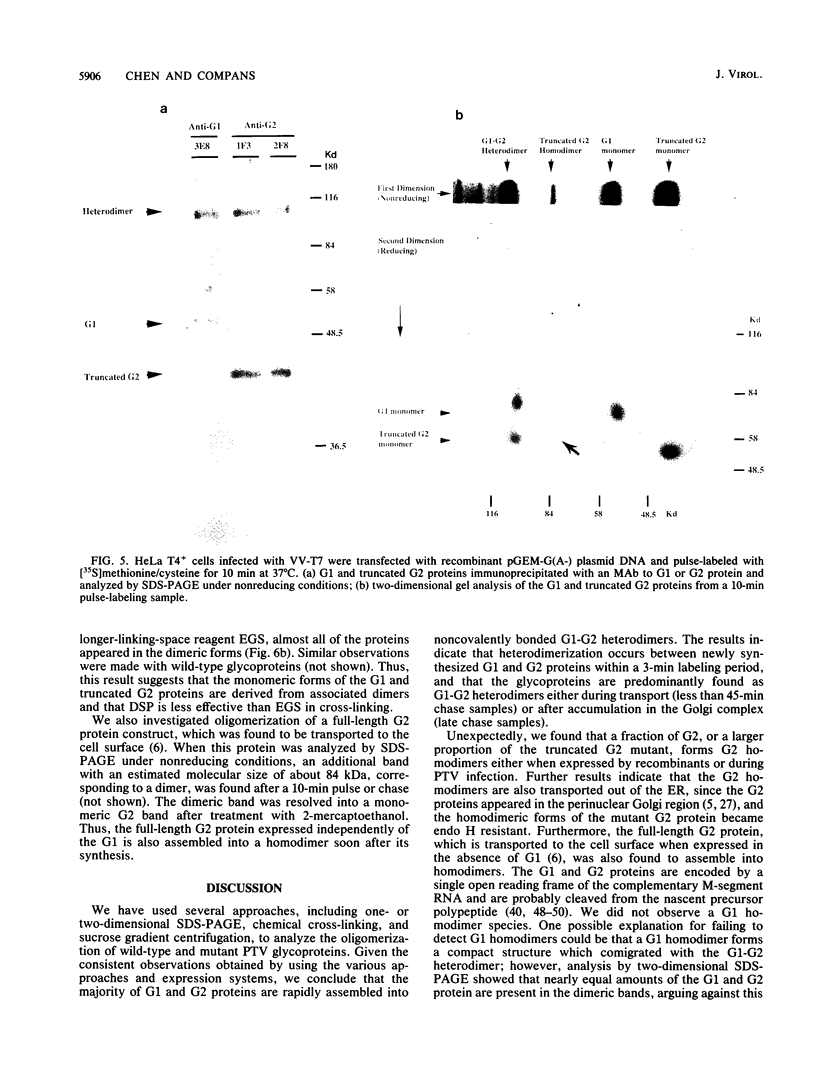
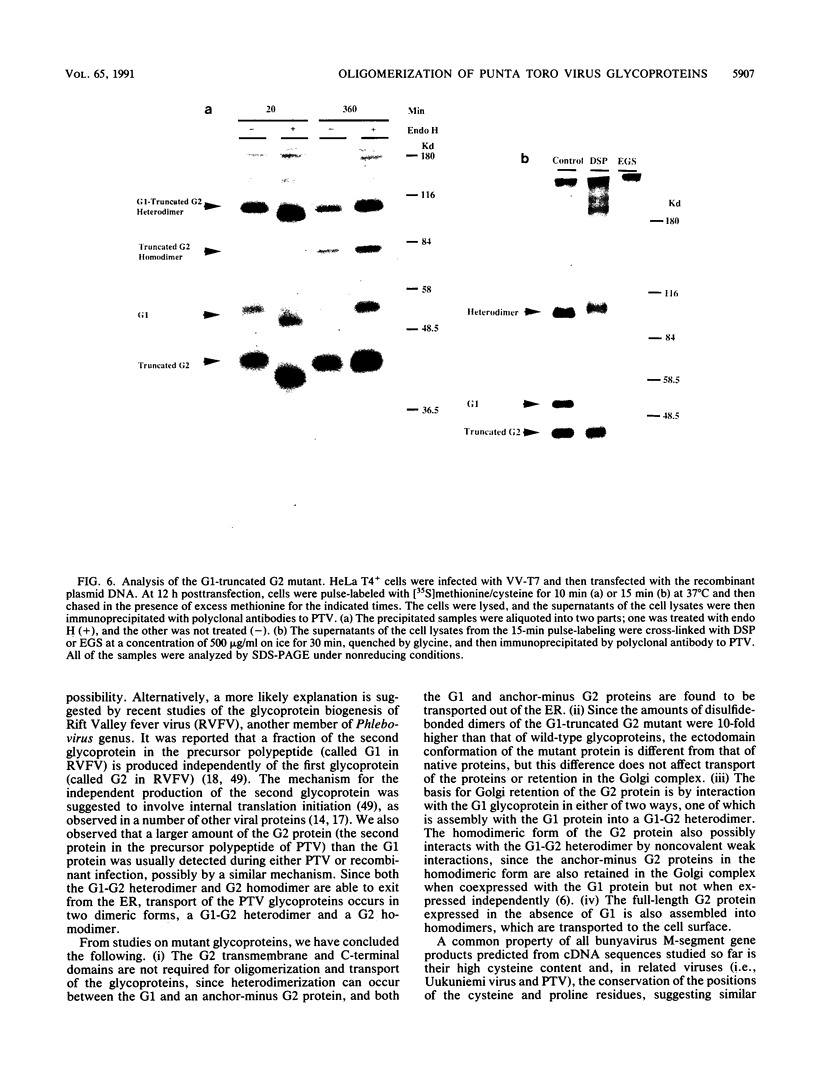


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Matsuoka Y., Compans R. W. Assembly and polarized release of Punta Toro virus and effects of brefeldin A. J Virol. 1991 Mar;65(3):1427–1439. doi: 10.1128/jvi.65.3.1427-1439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Matsuoka Y., Compans R. W. Golgi complex localization of the Punta Toro virus G2 protein requires its association with the G1 protein. Virology. 1991 Jul;183(1):351–365. doi: 10.1016/0042-6822(91)90148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley K. J., Lee E. U., Adler B., Browne J. K., Paulson J. C. Conversion of a Golgi apparatus sialyltransferase to a secretory protein by replacement of the NH2-terminal signal anchor with a signal peptide. J Biol Chem. 1989 Oct 25;264(30):17619–17622. [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M. Molecular biology of the Bunyaviridae. J Gen Virol. 1990 Mar;71(Pt 3):501–522. doi: 10.1099/0022-1317-71-3-501. [DOI] [PubMed] [Google Scholar]
- Eshita Y., Ericson B., Romanowski V., Bishop D. H. Analyses of the mRNA transcription processes of snowshoe hare bunyavirus S and M RNA species. J Virol. 1985 Sep;55(3):681–689. doi: 10.1128/jvi.55.3.681-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Internal initiation of translation on the vesicular stomatitis virus phosphoprotein mRNA yields a second protein. J Virol. 1986 Jun;58(3):797–804. doi: 10.1128/jvi.58.3.797-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara T., Smith J., Dalrymple J. M., Bishop D. H. Complete sequences of the glycoproteins and M RNA of Punta Toro phlebovirus compared to those of Rift Valley fever virus. Virology. 1985 Jul 15;144(1):246–259. doi: 10.1016/0042-6822(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakach L. T., Suzich J. A., Collett M. S. Rift Valley fever virus M segment: phlebovirus expression strategy and protein glycosylation. Virology. 1989 Jun;170(2):505–510. doi: 10.1016/0042-6822(89)90442-x. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kuismanen E., Hedman K., Saraste J., Pettersson R. F. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982 Nov;2(11):1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E. Posttranslational processing of Uukuniemi virus glycoproteins G1 and G2. J Virol. 1984 Sep;51(3):806–812. doi: 10.1128/jvi.51.3.806-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff D. H., Lenard J. A membrane glycoprotein that accumulates intracellularly: cellular processing of the large glycoprotein of LaCrosse virus. Cell. 1982 Apr;28(4):821–829. doi: 10.1016/0092-8674(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y., Ihara T., Bishop D. H., Compans R. W. Intracellular accumulation of Punta Toro virus glycoproteins expressed from cloned cDNA. Virology. 1988 Nov;167(1):251–260. doi: 10.1016/0042-6822(88)90075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Bunyaviridae: morphologic and morphogenetic similarities of Bunyamwera serologic supergroup viruses and several other arthropod-borne viruses. Intervirology. 1973;1(4):297–316. doi: 10.1159/000148858. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Jackson M., Peterson P. A. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989 Aug 25;58(4):707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pensiero M. N., Jennings G. B., Schmaljohn C. S., Hay J. Expression of the Hantaan virus M genome segment by using a vaccinia virus recombinant. J Virol. 1988 Mar;62(3):696–702. doi: 10.1128/jvi.62.3.696-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R., Pettersson R. F. Formation and intracellular transport of a heterodimeric viral spike protein complex. J Cell Biol. 1991 Jan;112(2):257–266. doi: 10.1083/jcb.112.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Kuismanen E., Pettersson R. F. Monosaccharide sequence of protein-bound glycans of Uukuniemi virus. J Virol. 1982 Feb;41(2):390–400. doi: 10.1128/jvi.41.2.390-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Theiler F., Horvath S. J., Tomich J. M., Richards J. H., Allison D. S., Schatz G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988 Mar;7(3):649–653. doi: 10.1002/j.1460-2075.1988.tb02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Welch W. J., Sefton B. M., Esch F. S., Ling N. C. Vesicular stomatitis virus glycoprotein is anchored in the viral membrane by a hydrophobic domain near the COOH terminus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3884–3888. doi: 10.1073/pnas.77.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnholm R., Pettersson R. F. Complete nucleotide sequence of the M RNA segment of Uukuniemi virus encoding the membrane glycoproteins G1 and G2. Virology. 1987 Sep;160(1):191–202. doi: 10.1016/0042-6822(87)90060-2. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. S., Schmaljohn A. L., Dalrymple J. M. Hantaan virus M RNA: coding strategy, nucleotide sequence, and gene order. Virology. 1987 Mar;157(1):31–39. doi: 10.1016/0042-6822(87)90310-2. [DOI] [PubMed] [Google Scholar]
- Semenza J. C., Hardwick K. G., Dean N., Pelham H. R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990 Jun 29;61(7):1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Smith J. F., Pifat D. Y. Morphogenesis of sandfly viruses (Bunyaviridae family). Virology. 1982 Aug;121(1):61–81. doi: 10.1016/0042-6822(82)90118-0. [DOI] [PubMed] [Google Scholar]
- Stirzaker S. C., Both G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell. 1989 Mar 10;56(5):741–747. doi: 10.1016/0092-8674(89)90677-6. [DOI] [PubMed] [Google Scholar]
- Suzich J. A., Collett M. S. Rift Valley fever virus M segment: cell-free transcription and translation of virus-complementary RNA. Virology. 1988 Jun;164(2):478–486. doi: 10.1016/0042-6822(88)90562-4. [DOI] [PubMed] [Google Scholar]
- Suzich J. A., Kakach L. T., Collett M. S. Expression strategy of a phlebovirus: biogenesis of proteins from the Rift Valley fever virus M segment. J Virol. 1990 Apr;64(4):1549–1555. doi: 10.1128/jvi.64.4.1549-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Seppälä P., Pettersson R. F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981 Jan;37(1):72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmoen T. L., Kakach L. T., Collett M. S. Rift Valley fever virus M segment: cellular localization of M segment-encoded proteins. Virology. 1988 Sep;166(1):275–280. doi: 10.1016/0042-6822(88)90174-2. [DOI] [PubMed] [Google Scholar]