Abstract
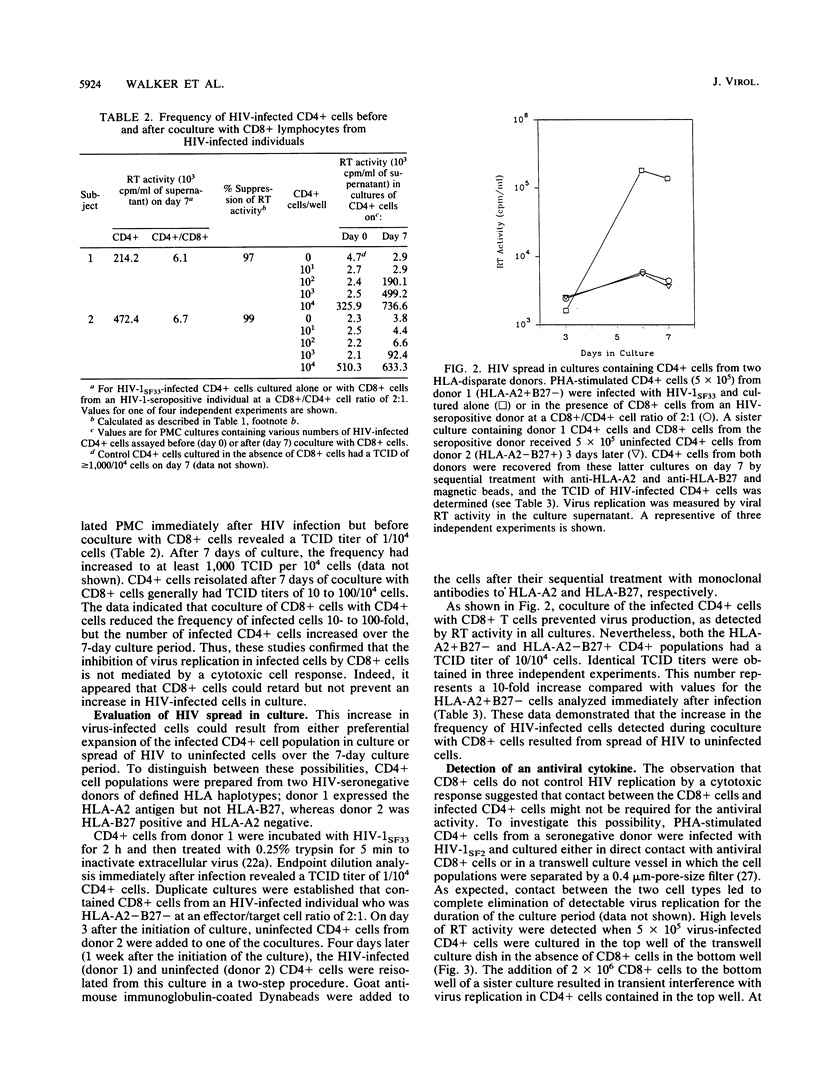
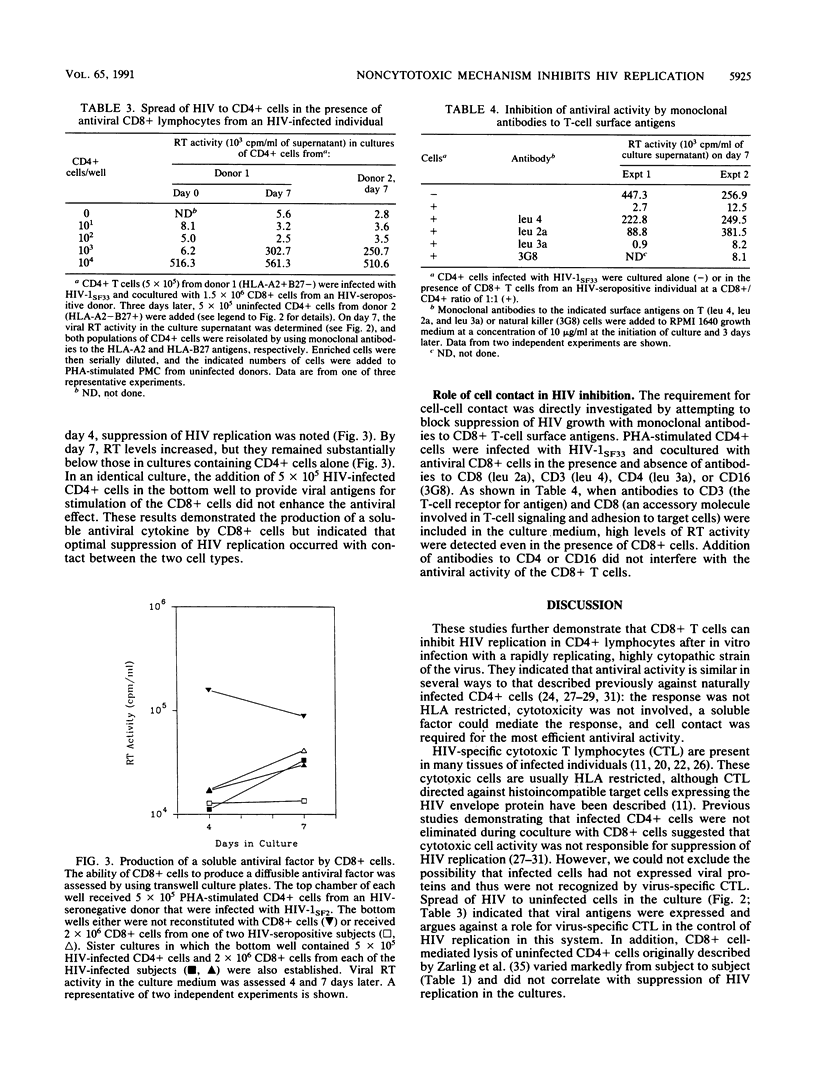
The mechanism by which CD8+ T cells from human immunodeficiency virus (HIV)-infected individuals suppress HIV replication in acutely infected CD4+ T cells was investigated. Cytotoxicity was not involved, as the antiviral activity of the CD8+ cells did not correlate with the ability to lyse HIV-infected or uninfected CD4+ T cells. In addition, the frequency of HIV-infected CD4+ cells increased during coculture with CD8+ T cells even in the absence of detectable levels of virus replication. Moreover, separation of the CD4+ and CD8+ cells by a 0.4-micron-pore-size filter delayed HIV replication, indicating a role, at least in part, for a soluble factor. However, cell contact was required for optimal antiviral activity. These results extend further the observation on the mechanism of antiviral HIV activity by CD8+ cells from infected individuals. They support the conclusion that CD8+ cells can play a major role in preventing development of disease in HIV-infected individuals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinchmann J. E., Gaudernack G., Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990 Apr 15;144(8):2961–2966. [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Castro B. A., Weiss C. D., Wiviott L. D., Levy J. A. Optimal conditions for recovery of the human immunodeficiency virus from peripheral blood mononuclear cells. J Clin Microbiol. 1988 Nov;26(11):2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Andrus L., Steinman R. M. Lymphokine and nonlymphokine mRNA levels in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1986 Apr 1;163(4):922–937. doi: 10.1084/jem.163.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Chalifoux L. V., Lord C. I., Letvin N. L. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988 Apr 1;140(7):2237–2242. [PubMed] [Google Scholar]
- Kannagi M., Masuda T., Hattori T., Kanoh T., Nasu K., Yamamoto N., Harada S. Interference with human immunodeficiency virus (HIV) replication by CD8+ T cells in peripheral blood leukocytes of asymptomatic HIV carriers in vitro. J Virol. 1990 Jul;64(7):3399–3406. doi: 10.1128/jvi.64.7.3399-3406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkhabwala M., Sehajpal P., Skolnik E., Smith D., Sharma V. K., Vlassara H., Cerami A., Suthanthiran M. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med. 1990 Mar 1;171(3):941–946. doi: 10.1084/jem.171.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Earl P., Powell D., Pantaleo G., Merli S., Moss B., Fauci A. S. Group-specific, major histocompatibility complex class I-restricted cytotoxic responses to human immunodeficiency virus 1 (HIV-1) envelope proteins by cloned peripheral blood T cells from an HIV-1-infected individual. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8638–8642. doi: 10.1073/pnas.85.22.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Tobler L. H., McHugh T. M., Casavant C. H., Stites D. P. Long-term cultivation of T-cell subsets from patients with acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1985 Jun;35(3):328–336. doi: 10.1016/0090-1229(85)90093-5. [DOI] [PubMed] [Google Scholar]
- Margolick J. B., Volkman D. J., Folks T. M., Fauci A. S. Amplification of HTLV-III/LAV infection by antigen-induced activation of T cells and direct suppression by virus of lymphocyte blastogenic responses. J Immunol. 1987 Mar 15;138(6):1719–1723. [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Mikovits J. A., Raziuddin, Gonda M., Ruta M., Lohrey N. C., Kung H. F., Ruscetti F. W. Negative regulation of human immune deficiency virus replication in monocytes. Distinctions between restricted and latent expression in THP-1 cells. J Exp Med. 1990 May 1;171(5):1705–1720. doi: 10.1084/jem.171.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984 Mar;132(3):1410–1415. [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Poli G., Orenstein J. M., Kinter A., Folks T. M., Fauci A. S. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989 May 5;244(4904):575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Näher H., Stroehmann I. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature. 1988 Sep 8;335(6186):178–181. doi: 10.1038/335178a0. [DOI] [PubMed] [Google Scholar]
- Tang S. B., Levy J. A. Inactivation of HIV-1 by trypsin and its use in demonstrating specific virus infection of cells. J Virol Methods. 1991 Jun;33(1-2):39–46. doi: 10.1016/0166-0934(91)90005-k. [DOI] [PubMed] [Google Scholar]
- Tateno M., Levy J. A. MT-4 plaque formation can distinguish cytopathic subtypes of the human immunodeficiency virus (HIV). Virology. 1988 Nov;167(1):299–301. doi: 10.1016/0042-6822(88)90084-0. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Levy J. A. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology. 1989 Apr;66(4):628–630. [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ T lymphocyte control of HIV replication in cultured CD4+ cells varies among infected individuals. Cell Immunol. 1989 Apr 1;119(2):470–475. doi: 10.1016/0008-8749(89)90259-1. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Wiviott L. D., Walker C. M., Levy J. A. CD8+ lymphocytes suppress HIV production by autologous CD4+ cells without eliminating the infected cells from culture. Cell Immunol. 1990 Jul;128(2):628–634. doi: 10.1016/0008-8749(90)90054-u. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Krowka J. F., Stites D. P., Goeddel D. V. In vitro anti-human immunodeficiency virus activities of tumor necrosis factor-alpha and interferon-gamma. J Immunol. 1988 Jan 1;140(1):120–124. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R. S., Ware C. F., Granger G. A. The human LT system. XI. Identification of LT and "TNF-like" forms from stimulated natural killers, specific and nonspecific cytotoxic human T cells in vitro. J Immunol. 1986 Sep 15;137(6):1878–1884. [PubMed] [Google Scholar]
- Zarling J. M., Ledbetter J. A., Sias J., Fultz P., Eichberg J., Gjerset G., Moran P. A. HIV-infected humans, but not chimpanzees, have circulating cytotoxic T lymphocytes that lyse uninfected CD4+ cells. J Immunol. 1990 Apr 15;144(8):2992–2998. [PubMed] [Google Scholar]
- van de Griend R. J., Bolhuis R. L., Stoter G., Roozemond R. C. Regulation of cytolytic activity in CD3- and CD3+ killer cell clones by monoclonal antibodies (anti-CD16, anti-CD2, anti-CD3) depends on subclass specificity of target cell IgG-FcR. J Immunol. 1987 May 15;138(10):3137–3144. [PubMed] [Google Scholar]



