Abstract
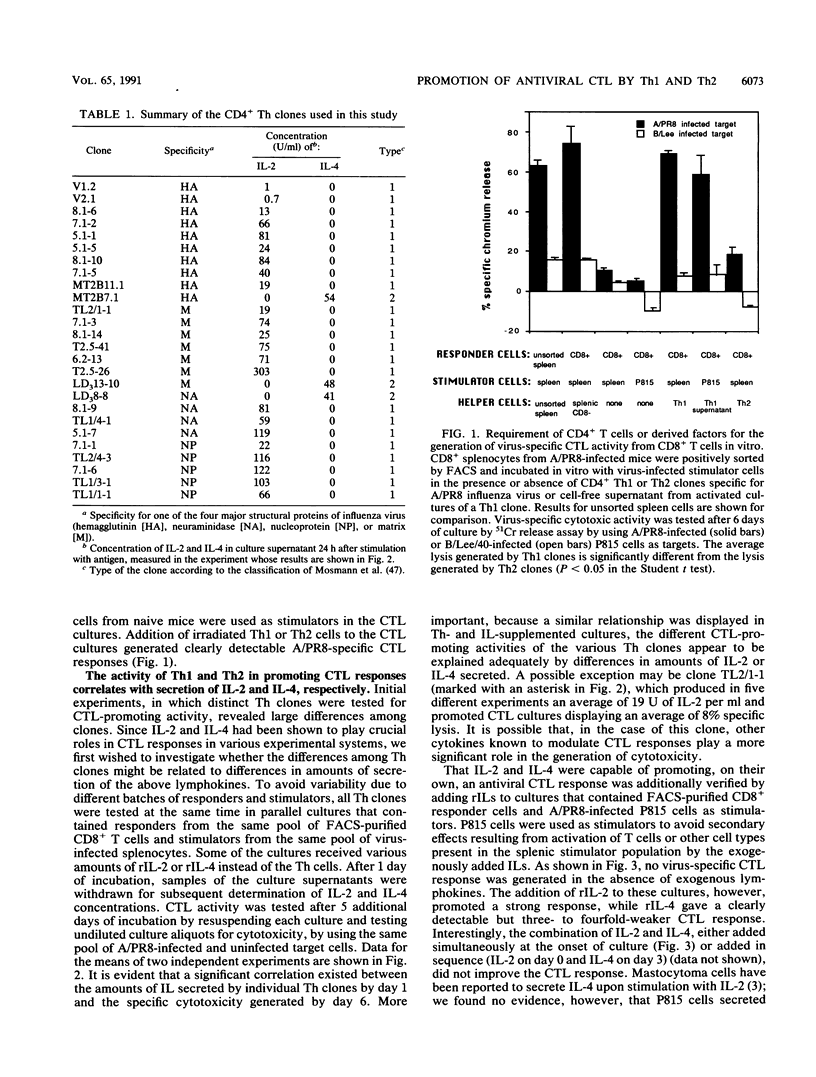
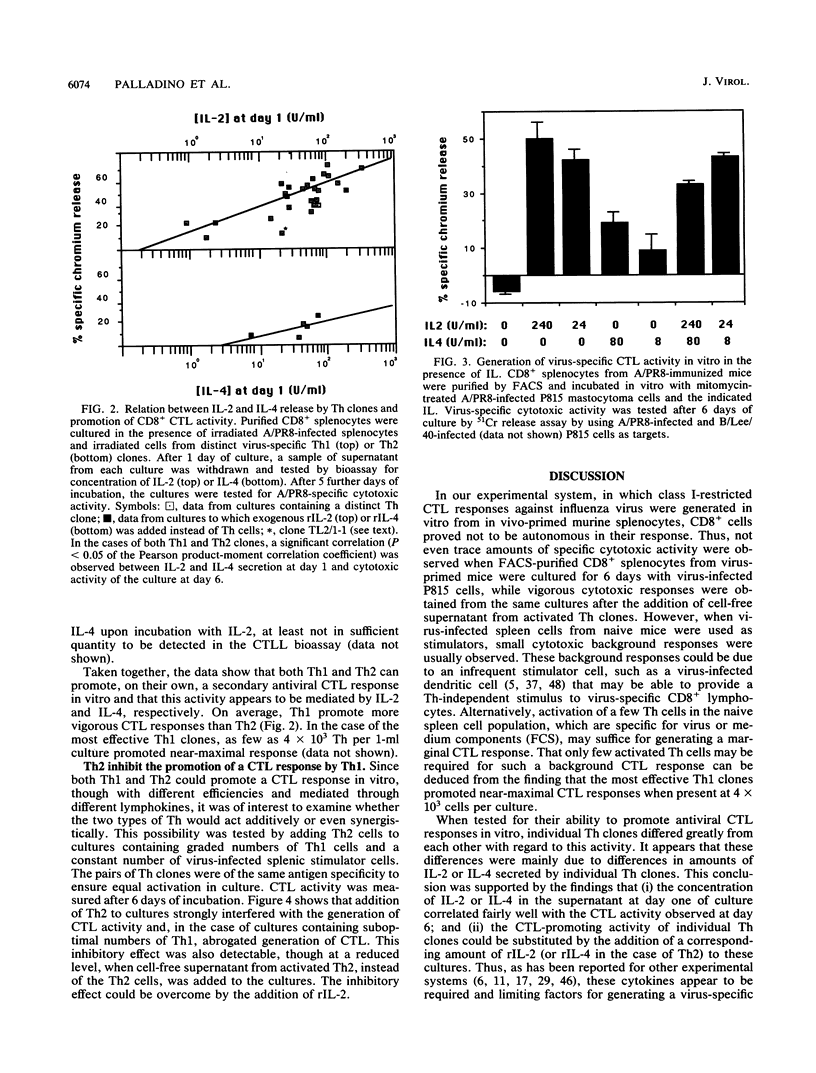
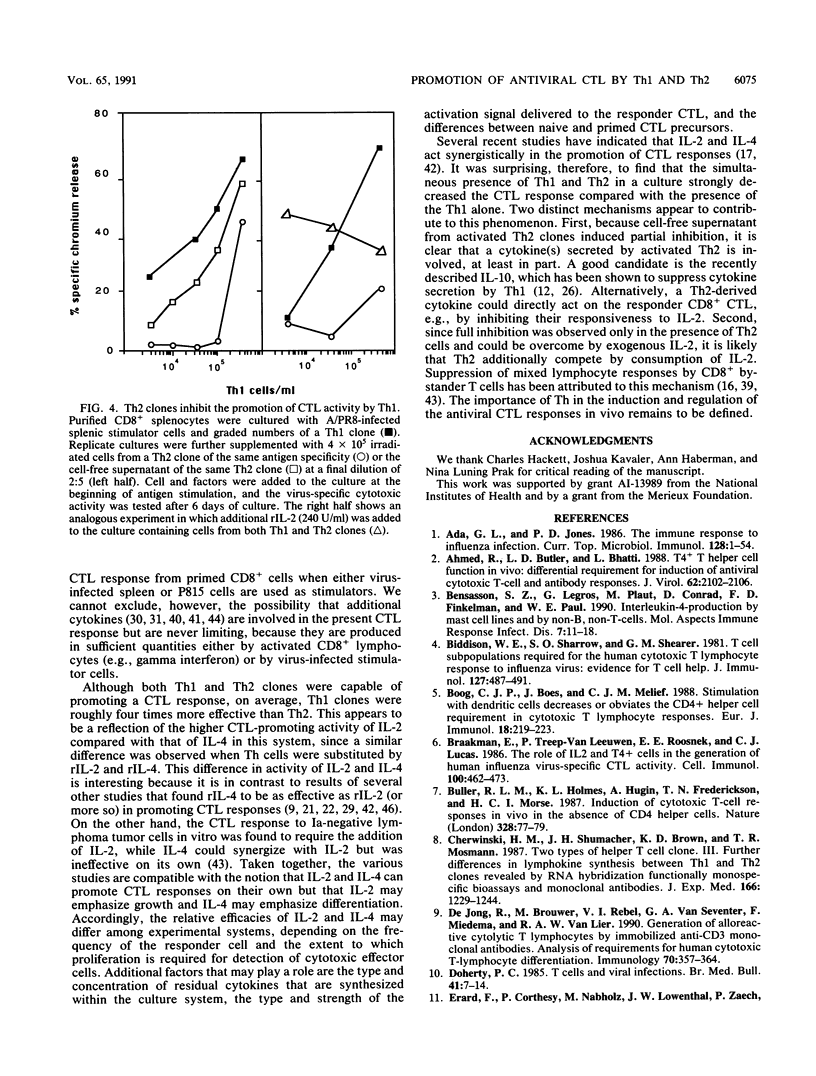
The activity of distinct CD4+ T-helper cell (Th) clones in promoting secondary A/PR/8/34/Mt.S.(H1N1) (A/PR8) influenza virus-specific, class I-restricted cytotoxic T-lymphocyte (CTL) responses in vitro was examined. CD8+ T cells which had been purified by fluorescence-activated cell sorter from spleen cells of A/PR8-primed mice were used as responders. On their own, purified CD8+ T cells were unable to generate cytotoxic activity upon in vitro culture with A/PR8-infected stimulator cells. Significant cytotoxic activity was generated in cultures that were additionally supplemented with A/PR8-specific Th clones or cell-free supernatant from these clones. Although there were large differences among individual Th clones in this function, Th clones of type 1 (Th1) promoted, on average, significantly stronger cytotoxic responses than Th clones of type 2 (Th2). The differences in promotion of a cytotoxic response correlated with the amount of interleukin-2 (IL-2) or IL-4 secreted by individual Th clones. These two lymphokines accounted for the CTL-promoting activity of the respective Th clones, since addition of recombinant IL-2 (IL-2) or rIL-4 to Th-free cultures substituted fully for the respective Th clones. As observed with Th clones, rIL-2 was significantly more effective than rIL-4 in promoting a cytotoxic response. When used in combination, Th2 clones had an antagonistic effect on the generation of a CTL response by Th1 clones. This effect could be partially transferred with cell-free supernatant from activated Th2 clones and could be reversed by addition of excess rIL-2. Both consumption of IL-2 by Th2 and secretion of an inhibitory factor(s) appear to be involved in this phenomenon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Jones P. D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- Ahmed R., Butler L. D., Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988 Jun;62(6):2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddison W. E., Sharrow S. O., Shearer G. M. T cell subpopulations required for the human cytotoxic T lymphocyte response to influenza virus: evidence for T cell help. J Immunol. 1981 Aug;127(2):487–491. [PubMed] [Google Scholar]
- Boog C. J., Boes J., Melief C. J. Stimulation with dendritic cells decreases or obviates the CD4+ helper cell requirement in cytotoxic T lymphocyte responses. Eur J Immunol. 1988 Feb;18(2):219–223. doi: 10.1002/eji.1830180206. [DOI] [PubMed] [Google Scholar]
- Braakman E., Treep-Van Leeuwen P., Roosnek E. E., Lucas C. J. The role of IL-2 and T4+ cells in the generation of human influenza virus-specific CTL activity. Cell Immunol. 1986 Jul;100(2):462–473. doi: 10.1016/0008-8749(86)90045-6. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R., Brouwer M., Rebel V. I., Van Seventer G. A., Miedema F., Van Lier R. A. Generation of alloreactive cytolytic T lymphocytes by immobilized anti-CD3 monoclonal antibodies. Analysis of requirements for human cytolytic T-lymphocyte differentiation. Immunology. 1990 Jul;70(3):357–364. [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C. T cells and viral infections. Br Med Bull. 1985 Jan;41(1):7–14. doi: 10.1093/oxfordjournals.bmb.a072028. [DOI] [PubMed] [Google Scholar]
- Erard F., Corthesy P., Nabholz M., Lowenthal J. W., Zaech P., Plaetinck G., MacDonald H. R. Interleukin 2 is both necessary and sufficient for the growth and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Immunol. 1985 Mar;134(3):1644–1652. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishwild D. M., Benike C. J., Engleman E. G. Activation of HLA-restricted EBV-specific cytotoxic T cells does not require CD4+ (helper) T cells or exogenous cytokines. J Immunol. 1988 Mar 15;140(6):1994–1998. [PubMed] [Google Scholar]
- Gerhard W., Hackett C., Melchers F. The recognition specificity of a murine helper T cell for hemagglutinin of influenza virus A/PR/8/34. J Immunol. 1983 May;130(5):2379–2385. [PubMed] [Google Scholar]
- Guerne P. A., Piguet P. F., Vassalli P. Production of interleukin 2, interleukin 3, and interferon by mouse T lymphocyte clones of Lyt-2+ and -2- phenotype. J Immunol. 1984 Apr;132(4):1869–1871. [PubMed] [Google Scholar]
- Günther J., Haas W., Von Boehmer H. Suppression of T cell responses through competition for T cell growth factor (interleukin 2). Eur J Immunol. 1982 Mar;12(3):247–249. doi: 10.1002/eji.1830120315. [DOI] [PubMed] [Google Scholar]
- Horohov D. W., Crim J. A., Smith P. L., Siegel J. P. IL-4 (B cell-stimulatory factor 1) regulates multiple aspects of influenza virus-specific cell-mediated immunity. J Immunol. 1988 Dec 15;141(12):4217–4223. [PubMed] [Google Scholar]
- Jennings S. R., Bonneau R. H., Smith P. M., Wolcott R. M., Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991 Mar;133(1):234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Rolink A., Melchers F. Recombinant interleukin 2 or 5, but not 3 or 4, induces maturation of resting mouse B lymphocytes and propagates proliferation of activated B cell blasts. J Exp Med. 1988 Apr 1;167(4):1377–1390. doi: 10.1084/jem.167.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Miethke T., Schmidberger R., Heeg K., Gillis S., Wagner H. Interleukin 4 (BSF-1) induces growth in resting murine CD8 T cells triggered via cross-linking of T3 cell surface structures. Eur J Immunol. 1988 May;18(5):767–772. doi: 10.1002/eji.1830180517. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Hooton J. W., Gillis S., Paetkau V. IL-4 potentiates the IL-2-dependent proliferation of mouse cytotoxic T cells. J Immunol. 1990 Feb 15;144(4):1331–1337. [PubMed] [Google Scholar]
- Mizuochi T., Hügin A. W., Morse H. C., 3rd, Singer A., Buller R. M. Role of lymphokine-secreting CD8+ T cells in cytotoxic T lymphocyte responses against vaccinia virus. J Immunol. 1989 Jan 1;142(1):270–273. [PubMed] [Google Scholar]
- Mizuochi T., Ono S., Malek T. R., Singer A. Characterization of two distinct primary T cell populations that secrete interleukin 2 upon recognition of class I or class II major histocompatibility antigens. J Exp Med. 1986 Mar 1;163(3):603–619. doi: 10.1084/jem.163.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., McKenzie D. T., Swain S. L., Dutton R. W. B cell stimulatory factor 1 (interleukin 4) is sufficient for the proliferation and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Exp Med. 1987 Nov 1;166(5):1464–1470. doi: 10.1084/jem.166.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Bevan M. J. A differentiation factor required for the expression of cytotoxic T-cell function. Nature. 1982 Apr 22;296(5859):754–757. doi: 10.1038/296754a0. [DOI] [PubMed] [Google Scholar]
- Renauld J. C., Vink A., Van Snick J. Accessory signals in murine cytolytic T cell responses. Dual requirement for IL-1 and IL-6. J Immunol. 1989 Sep 15;143(6):1894–1898. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Differential ability of B cells specific for external vs. internal influenza virus proteins to respond to help from influenza virus-specific T-cell clones in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4446–4450. doi: 10.1073/pnas.85.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Munitz T. I., Golding H., Rosenberg A. S., Mizuochi T. Recognition requirements for the activation, differentiation and function of T-helper cells specific for class I MHC alloantigens. Immunol Rev. 1987 Aug;98:143–170. doi: 10.1111/j.1600-065x.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Antigen-presenting cells for unprimed T cells. Immunol Today. 1989 Jan;10(1):17–23. doi: 10.1016/0167-5699(89)90060-1. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Capacity of purified Lyt-2+ T cells to mount primary proliferative and cytotoxic responses to Ia- tumour cells. Nature. 1986 Aug 7;322(6079):541–544. doi: 10.1038/322541a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Susskind B. M., Merluzzi V. J., Faanes R. B., Palladino M. A., Choi Y. S. Regulatory mechanisms in cytotoxic T lymphocyte development. I. A suppressor T cell subset that regulates the proliferative stage of CTL development. J Immunol. 1983 Feb;130(2):527–532. [PubMed] [Google Scholar]
- Takai Y., Herrmann S. H., Greenstein J. L., Spitalny G. L., Burakoff S. J. Requirement for three distinct lymphokines for the induction of cytotoxic T lymphocytes from thymocytes. J Immunol. 1986 Dec 1;137(11):3494–3500. [PubMed] [Google Scholar]
- Takatsu K., Kikuchi Y., Takahashi T., Honjo T., Matsumoto M., Harada N., Yamaguchi N., Tominaga A. Interleukin 5, a T-cell-derived B-cell differentiation factor also induces cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4234–4238. doi: 10.1073/pnas.84.12.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenn G., Takayama H., Hu-Li J., Paul W. E., Sitkovsky M. V. B cell stimulatory factor 1 (IL-4) enhances the development of cytotoxic T cells from Lyt-2+ resting murine T lymphocytes. J Immunol. 1988 Feb 15;140(4):1101–1106. [PubMed] [Google Scholar]
- Wabuke-Bunoti M. A., Taku A., Garman R., Fan D. P. Stimulation of anti-influenza cytolytic T lymphocytes by a synthetic peptide of the influenza hemagglutinin can be modulated by at least three independent helper factors. J Immunol. 1984 Oct;133(4):2186–2193. [PubMed] [Google Scholar]
- Widmer M. B., Bach F. H. Antigen-driven helper cell-independent cloned cytolytic T lymphocytes. Nature. 1981 Dec 24;294(5843):750–752. doi: 10.1038/294750a0. [DOI] [PubMed] [Google Scholar]
- Widmer M. B., Grabstein K. H. Regulation of cytolytic T-lymphocyte generation by B-cell stimulatory factor. Nature. 1987 Apr 23;326(6115):795–798. doi: 10.1038/326795a0. [DOI] [PubMed] [Google Scholar]
- Wysocka M., Bennink J. R. Limiting dilution analysis of memory cytotoxic T lymphocytes specific for individual influenza virus gene products. Cell Immunol. 1988 Apr 1;112(2):425–429. doi: 10.1016/0008-8749(88)90311-5. [DOI] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper T cells. J Exp Med. 1990 Apr 1;171(4):1315–1332. doi: 10.1084/jem.171.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Kisielow P., Leiserson W., Haas W. Lyt-2- T cell-independent functions of Lyt-2+ cells stimulated with antigen or concanavalin A. J Immunol. 1984 Jul;133(1):59–64. [PubMed] [Google Scholar]


