Abstract
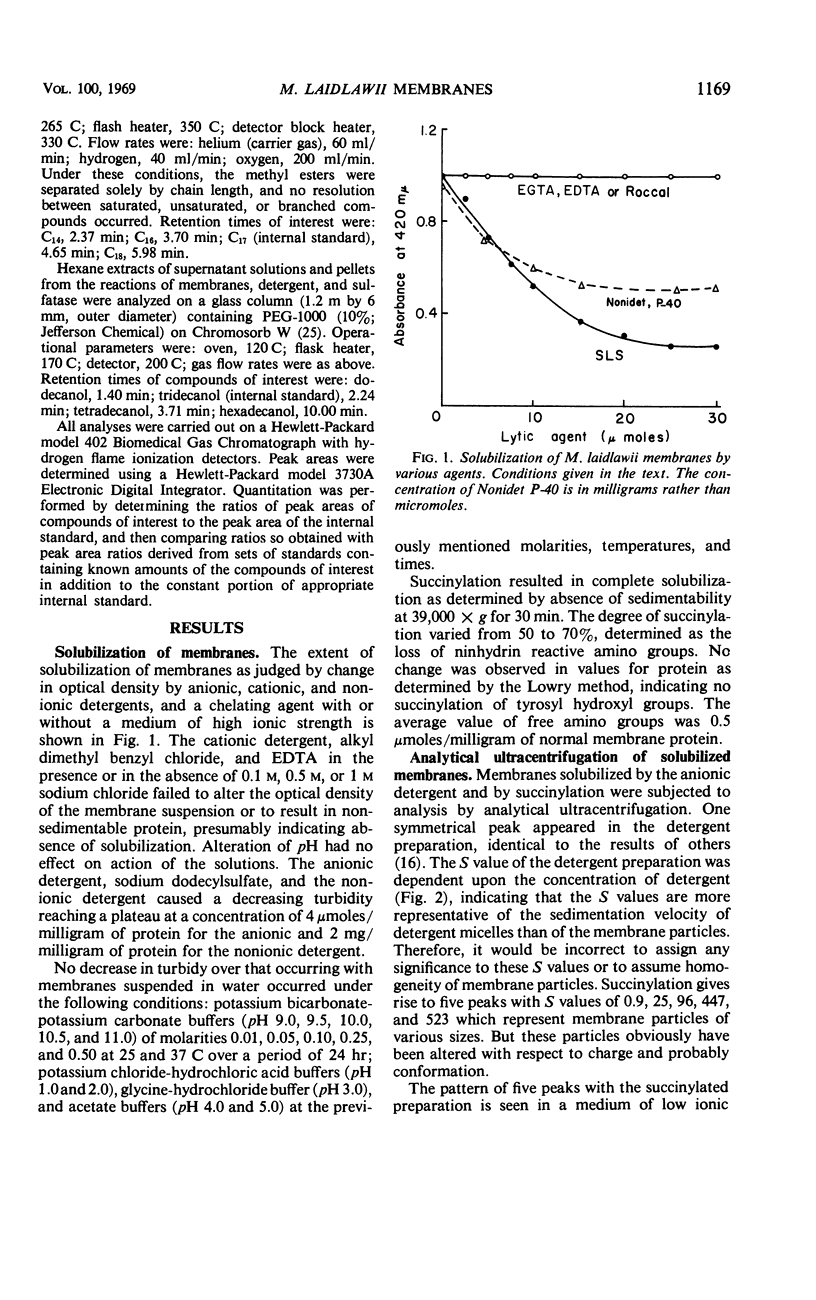
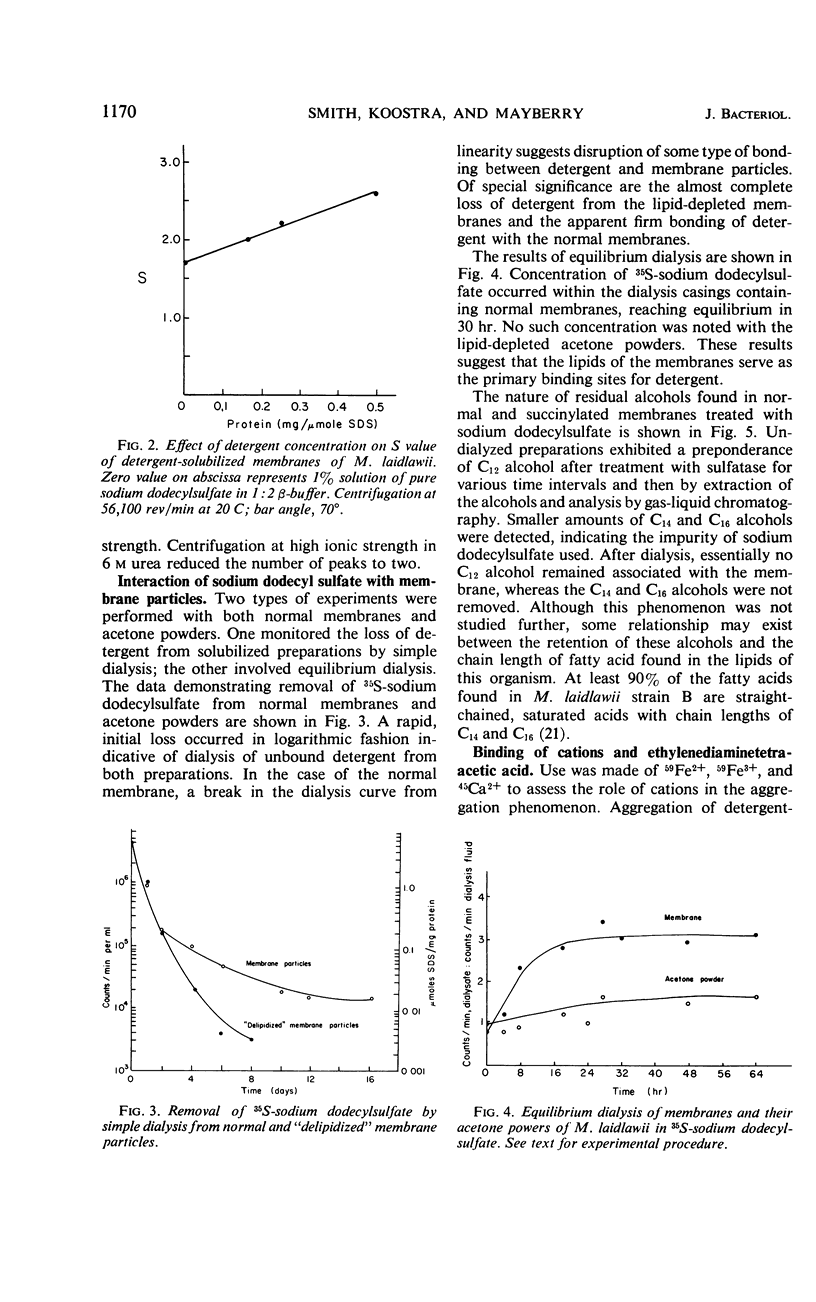
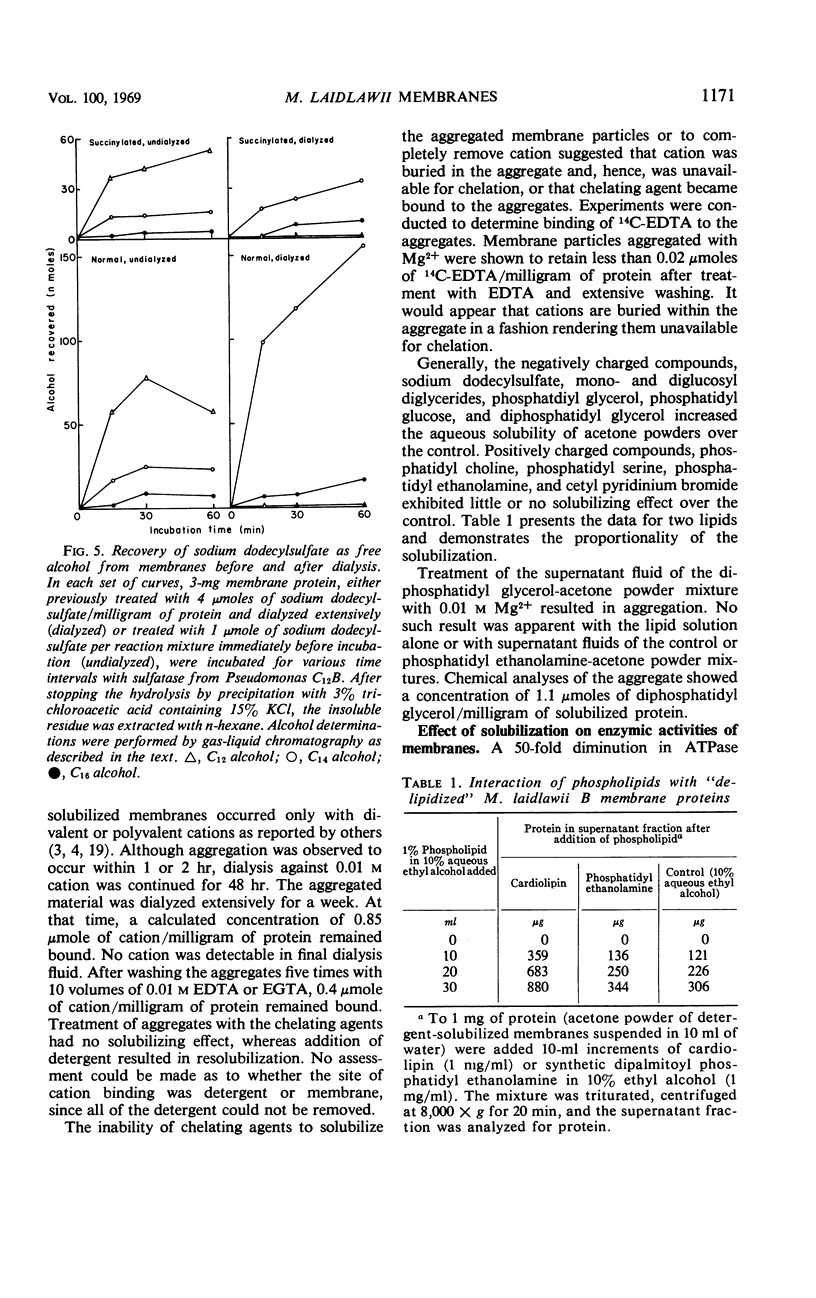
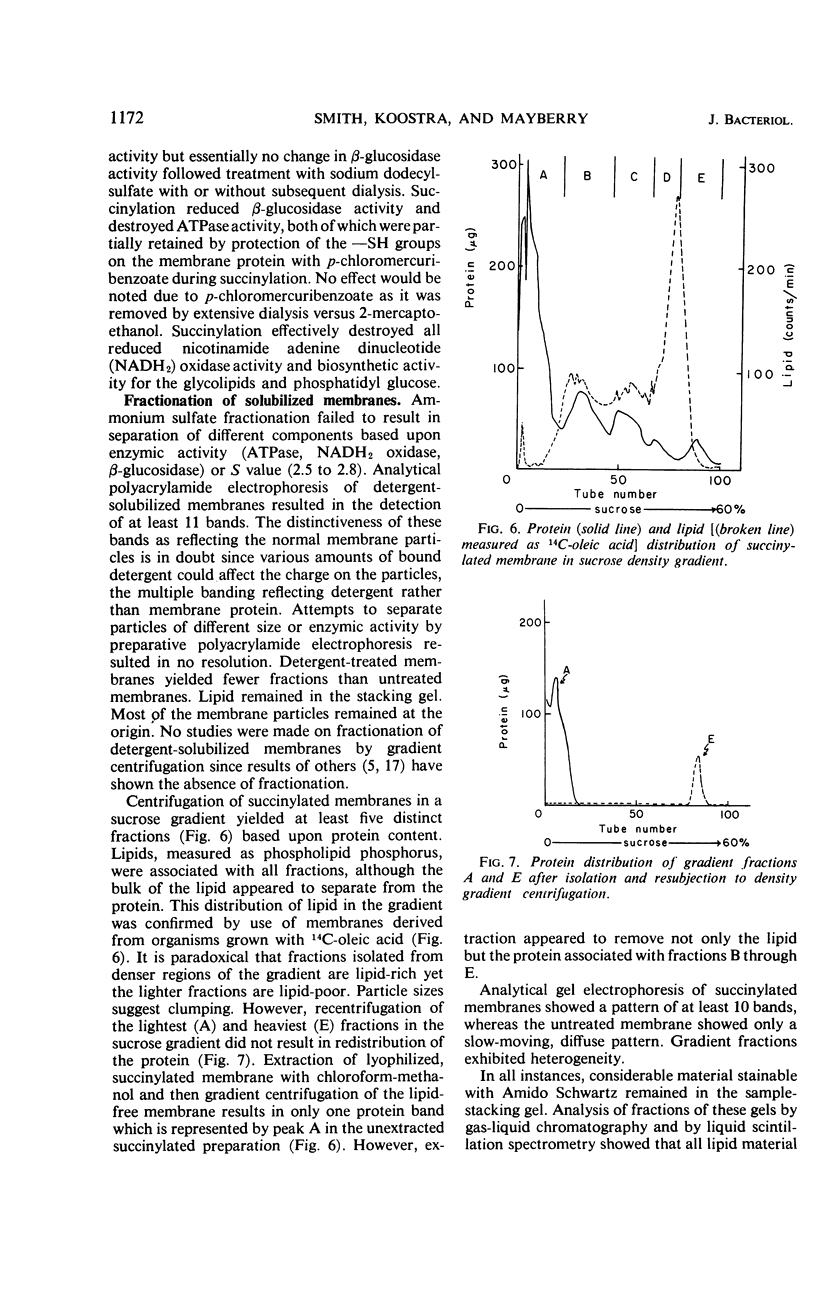
The cytoplasmic membrane of Mycoplasma laidlawii strain B is solubilized by anionic and nonionic detergents, succinylation, phospholipase A, alkaline phosphatase, trypsin, and chymotrypsin. Cationic detergents are without effect, as are chelating agents, even in the presence of high concentrations of monovalent cation. The detergent-solubilized membrane exhibits one peak in the analytical ultracentrifuge, but the sedimentation coefficient is dependent upon concentration of detergent. Simple dialysis does not remove all of the sodium dodecylsulfate except from lipid-depleted membrane particles. Membranes bind sodium dodecylsulfate but acetone powders of membranes do not. Sulfated alcohols with chain lengths of C14 and C16 are more tightly bound than dodecylsulfate. A constant amount of di- and trivalent cation is bound by the membrane upon aggregation. Only a portion of this cation is removable with chelating agents. No chelating agent is bound by these aggregates. A portion of the lipid-depleted membrane particles is solubilized by negatively charged lipids and detergents, giving rise to aggregates in the presence of divalent cation. Fractionations of detergent-solubilized membranes by preparative gel electrophoresis and ammonium sulfate were inconclusive. Density gradient centrifugation of succinylated membranes yielded at least five fractions which exhibited homogeneity by ultracentrifugation. Analytical gel electrophoresis of these fractions demonstrated heterogeneity. The composition of these five fractions suggested separation of protein from lipid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWSON R. M., BANGHAM A. D. The activation of surface films of lecithin by amphipathic molecules. Biochem J. 1959 Jul;72:493–496. doi: 10.1042/bj0720493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. 3. The formation and aggregation of small lipoprotein structures derived from sodium dodecyl sulfate-solubilized membrane components. Biochim Biophys Acta. 1968 Apr 29;150(3):376–384. doi: 10.1016/0005-2736(68)90136-3. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. IV. Structure and composition of membrane and aggregated components. Biochim Biophys Acta. 1968 Apr 29;150(3):385–396. doi: 10.1016/0005-2736(68)90137-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Terry T. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. I. Sodium dodecyl sulfate solubilization. Biochim Biophys Acta. 1967 Jul 3;135(3):381–390. doi: 10.1016/0005-2736(67)90028-4. [DOI] [PubMed] [Google Scholar]
- HASS L. F. ALDOLASE DISSOCIATION INTO SUBUNITS BY REACTION WITH SUCCINIC ANHYDRIDE. Biochemistry. 1964 Apr;3:535–541. doi: 10.1021/bi00892a012. [DOI] [PubMed] [Google Scholar]
- HENRIKSON C. V., SMITH P. F. BETA-GLUCOSIDASE ACTIVITY IN MYCOPLASMA. J Gen Microbiol. 1964 Oct;37:73–80. doi: 10.1099/00221287-37-1-73. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Ottolenghi A. C. Phospholipase C from Bacillus cereus, a zinc-requiring metalloenzyme. Biochim Biophys Acta. 1965 Dec 2;106(3):510–518. doi: 10.1016/0005-2760(65)90067-6. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Williams J. P., Mayberry W. R. Primary alcohol sulfatase in a Pseudomonas species. Appl Microbiol. 1965 Sep;13(5):698–701. doi: 10.1128/am.13.5.698-701.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- SMITH P. F., HENDRIKSON C. V. GLUCOSE-CONTAINING PHOSPHOLIPIDS IN MYCOPLASMA LAIDLAWII, STRAIN B. J Lipid Res. 1965 Jan;6:106–111. [PubMed] [Google Scholar]
- Shaw N., Smith P. F., Koostra W. L. The lipid composition of Mycoplasma laidlawii strain B. Biochem J. 1968 Apr;107(3):329–333. doi: 10.1042/bj1070329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry T. M., Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. II. Modes of aggregation of solubilized membrane components. Biochim Biophys Acta. 1967 Jul 3;135(3):391–405. doi: 10.1016/0005-2736(67)90029-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J., PAYNE W. J. ENZYMES INDUCED IN A BACTERIUM BY GROWTH ON SODIUM DODECYL SULFATE. Appl Microbiol. 1964 Jul;12:360–362. doi: 10.1128/am.12.4.360-362.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Mayberry W. R., Payne W. J. Metabolism of linear alcohols with various chain lengths by a Pseudomonas species. Appl Microbiol. 1966 Mar;14(2):156–160. doi: 10.1128/am.14.2.156-160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



