Abstract
Superoxide anion (O2−) plays a key role in the endogenous suppression of endothelium-derived nitric oxide (NO) bioactivity and has been implicated in the development of hypertension. In previous studies, we found that O2− is produced predominantly in the adventitia of isolated rabbit aorta and acts as a barrier to NO. In the present studies, we characterize the enzyme responsible for O2− production in the adventitia and show that this enzyme is a constitutively active NADPH oxidase with similar composition as the phagocyte NADPH oxidase. Constitutive O2−-generating activity was localized to aortic adventitial fibroblasts and was enhanced by the potent vasoconstrictor angiotensin II. Immunohistochemistry of aortic sections demonstrated the presence of p22phox, gp91phox, p47phox, and p67phox localized exclusively in rabbit aortic adventitia, coincident with the site of staining for O2− production. Furthermore, immunodepletion of p67phox from adventitial fibroblast particulates resulted in the loss of NADPH oxidase activity, which could be restored by the addition of recombinant p67phox. Further study into the regulation of this adventitial source of O2− is important in elucidating the mechanisms regulating the bioactivity of NO and may contribute to our understanding of the pathogenesis of hypertension.
In recent years, reactive oxygen species (ROS) such as superoxide anion (O2−) have been shown to play a number of roles in the body. Phagocytic cells produce ROS as a primary host defense mechanism (1), whereas other cells utilize ROS as intracellular second messengers for a wide range of cellular functions. For example, ROS participate in Ras-mediated mitogenic signaling in fibroblasts as well as in leukocyte apoptosis (2, 3).
The phagocyte NADPH oxidase or respiratory burst oxidase is the best characterized ROS-generating system and is a multicomponent enzyme complex that catalyzes the one-electron reduction of oxygen to O2−. Its components include the two membrane-spanning polypeptides, p22phox and gp91phox, which comprise flavocytochrome b558, and three cytoplasmic polypeptides, p40phox, p47phox, and p67phox (4–6). Additionally, the cytosolic guanine nucleotide-binding protein Rac2 is required for oxidase activation (7). Exposure of the cell to a variety of agonists induces the association of the cytosolic with the membrane-associated components and causes activation of the normally dormant oxidase (4–6).
Several groups have shown that NAD(P)H oxidase(s) exist in nonphagocytic cells including carotid body (8), mesangial cells (9), vascular smooth muscle cells (10, 11), endothelial cells (12, 13), and fibroblasts (14). The NAD(P)H oxidase systems in these cells have not yet been well characterized, and even the substrate specificity of these oxidase(s) with regard to NADH and NADPH is still not clear (10–12, 15). In vascular smooth muscle cells of the bovine pulmonary artery and rat aorta, an NADH oxidase has been described, and in the rat aorta these cells express the mRNA for one of the flavocytochrome b558 subunits found in the neutrophil membranes, p22phox (10, 16). Likewise, endothelial cells of the bovine pulmonary artery (12) as well as the human umbilical vein, HUVEC (13), seem to contain a O2−-generating NADH oxidase; mRNA for all four major subunits of the phagocyte NAD(P)H oxidase (13) have been detected by amplification using reverse transcription-PCR. Yet, the localization of the major vascular NAD(P)H oxidase has not been addressed.
Previously, we reported that the rabbit aortic adventitia contains an NADPH-dependent oxidase (15). We also reported that the adventitia is the major site of O2− production in the rabbit aorta and recently found that the adventitia acts as a barrier for nitric oxide (17). Because of the potential importance of an aortic NADPH oxidase in the regulation of NO function and vascular physiology, we have characterized the aortic adventitial NADPH oxidase and show here that adventitial fibroblasts contain an NADPH oxidase that is very similar to the phagocyte enzyme in many regards except for its constitutive nature.
METHODS
Tissue Culture of Aortic Adventitial Fibroblasts.
Thoracic aortas were removed sterilely from male New Zealand White rabbits as previously described (15) and placed in DMEM/Ham’s F-12 medium containing 100 units/ml penicillin and 100 μg/ml streptomycin. Vessels were then cleared of adventitial adipose tissue, cut longitudinally, and scraped of endothelial cells using a gentle motion with forceps. Medial smooth muscle cells were peeled from the adventitia using forceps, and the adventitia was digested in DMEM/F12 medium containing 1 mg/ml collagenase, 0.125 mg/ml elastase for 4 h at 37°C. The resulting solution was then centrifuged for 5 min at 675 × g. The pellet was resuspended in 12 ml of DMEM/F12 medium containing 20% (vol/vol) fetal bovine serum plated on 100-mm culture dishes and allowed to reach confluence (4–5 days). These cells were considered Po cells. Confluent cells were passaged with 1:1 (vol/vol) PBS/trypsin–EDTA for 5 min at 37°C. Cells were resuspended in DMEM/F12 plus 20% fetal bovine serum and seeded at a density of 30% as described by Masur et al. (18) to ensure the growth of fibroblasts versus myofibroblasts. For measurements of basal NADPH oxidase activity, 95% confluent cells in the third passage were harvested as above, and particulate fractions were prepared (see below). We confirmed the presence of fibroblasts by morphologic and growth characteristics (19) and by the lack of α-actin staining (not shown).
For measurements of the effect of time and angiotensin II (AngII) on NADPH oxidase activity, the cells were cultured to the third passage and to 95% confluence, then made quiescent by serum deprivation to 0.67% fetal bovine serum for 48 h. At the end of this period, the cells were either added a vehicle control or AngII for various time periods. In the event that an inhibitor or antagonist was administered, it was administered 15 min before AngII or vehicle. Then, at the end of the incubation period, the cells were removed from the plate by trypsinization, and the procedure for preparation of particulates was performed.
Preparation of Particulate Fractions.
P3 cultured fibroblasts were harvested as described above, and the pellet was resuspended in 400 μl of ice-cold Tris–sucrose buffer containing (in mM): 10 Trizma base, 340 sucrose, 1 phenylmethylsulfonyl fluoride, 1 EDTA, and 10 μg/ml each of leupeptin, pepstatin, and aprotinin (pH 7.1). The cell suspension was sonicated by using four 15-s bursts. Because in early experiments a 3,000 × g centrifugation did not result in substantial activity in the pellet (see Table 1), the cell sonicate was centrifuged at 28,000 × g for 15 min at 4°C. The 28,000 × g pellet contained the most 5′-nucleotidase activity. This result is consistent with the activity being associated with plasma membranes (15). The supernatant was removed, and the pellet was rinsed with 400 μl of Tris–sucrose buffer. After another centrifugation at 28,000 × g for 15 min at 4°C, the pellet was resuspended in 100 μl of Tris–sucrose buffer and homogenized with a smooth glass mortar and Teflon pestle.
Table 1.
Subcellular fractionation of O2−-generating activity in sonicates of aortic adventitial fibroblasts
| Fraction | Volume, ml | Protein, mg/ml | Specific activity, nmol/min/mg | Total activity, nmol/min |
|---|---|---|---|---|
| Sonicate | 0.5 | 0.33 | 0.92 | 0.15 |
| (6.44) | (1.06) | |||
| 3,000 × g | 0.2 | 0.07 | 1.62 | 0.02 |
| pellet | (4.61) | (0.06) | ||
| 28,000 × g | 0.2 | 0.40 | 2.56 | 0.21 |
| pellet | (37.8) | (3.02) | ||
| Supernatant | 0.2 | 0.73 | 0.16 | 0.02 |
| (0.55) | (0.08) |
For specific and total activities, numbers not in parentheses are for NADPH-dependent activities, and numbers in parentheses are for NADH-dependent activities (45). These data are representative of three experiments.
Measurement of NAD(P)H Oxidase Activity.
Approximately 10–20 μg of fibroblast particulate fraction was assayed for O2−-dependent lucigenin chemiluminescence as described previously (15). Units of chemiluminescence were converted to nmoles of O2− by standardizations with the xanthine oxidase–cytochrome c assay (15). To confirm that these measurements were not an artifact of the lucigenin assay, as has been reported by Liochev and Fridovich (50), we also directly measured O2− using cytochrome c as the electron acceptor and calculating O2− directly by use of the extinction coefficient. In brief, we quantified control and AngII-induced O2− in particulate fractions of fibroblasts using the cytochrome c assay as described by Pagano et al. (15) and found similar levels, if not higher, of NADPH-dependent O2− (18 ± 9.7 versus 30 ± 20 nmol/min/mg protein, respectively; n = 3). Although the means do exhibit a substantial variation, they are within range of the values compiled using lucigenin (see below). The data would suggest that lucigenin was not artifactually contributing to the generation of O2− under these conditions. Second, we added lucigenin (250 μM; n = 3 preparations) in some cases to the cytochrome c assay of particulate fraction and found no enhancement in superoxide dismutase-inhibitable cytochrome c reduction. From this we conclude that under the conditions of our assay, lucigenin is not generating O2− and is a more conservative and discerning quantitation of the difference between control and AngII-stimulated O2−.
Immunodepletion of p67phox.
Particulate fractions (10–15 μg) were incubated at 4°C with either 1:1000 dilutions of p67phox antibody or control isotype IgG 2a with a κ light chain for 2 h with gentle shaking. 100 μl of protein A Sepharose were then added to the incubations, and shaking was resumed for 45 min. The solution was poured over a column containing a frit to allow the unbound fraction (flow-through) to pass through and be collected. NADPH-dependent O2−-generating activity was measured in each fraction and compared with flow-through of control isotype antibody-treated samples unless otherwise indicated.
Immunoblotting.
Fibroblast particulate fractions were separated on 10–20% gradient SDS-polyacrylamide gels, transferred to poly(vinylidene difluoride) (PVDF), and Western blotted with a monoclonal antibody to p67phox (22). The blot was developed using Super Signal Ultra (Pierce).
Immunohistochemistry.
Thoracic aorta were removed sterilely from male New Zealand White rabbits, embedded in OCT compound, and snap-frozen on dry ice. Frozen aortas were cryosectioned at 5 μm. The sections were air-dried for 1 h, fixed in cold acetone for 10 min, air-dried again for 1 h, washed with blotto [5% skim milk + 0.1% Tween 20 in Dulbecco’s PBS, pH 7.6 (DPBS)], and blocked with 10% goat serum in blotto for 30 min. The sections were then incubated overnight with control nonimmune mouse serum or with previously characterized monoclonal antibodies specifically recognizing gp91phox (22), p22phox (22), p47phox (23), and p67phox (23) in DPBS containing 1% BSA, 0.1% Tween 20, 0.1% NaN3, and 1% goat serum. After washing with blotto, the sections were incubated for 60 min at 25°C with 5 nm gold-conjugated goat anti-mouse antibody (Zymed) diluted 1:50 in the same buffer. The sections were rinsed with H2O, developed using silver acetate enhancing in the dark (49), and counterstained by using nuclear fast red (Vector Laboratories).
RESULTS
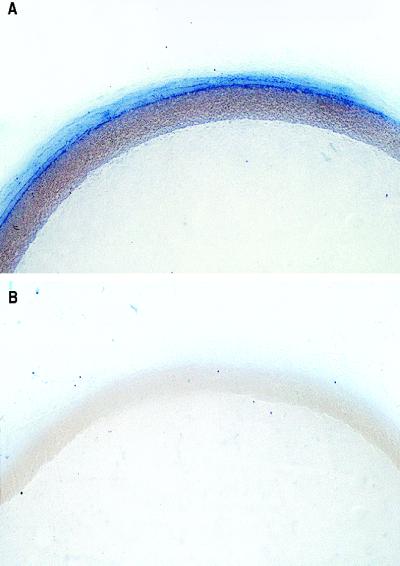
Previously, we reported that the adventitia of the rabbit aorta is the major site of O2− production in the particulate fraction (15). Fig. 1A confirms the site-specific production of O2− using nitroblue tetrazolium as a detector of O2− in an aorta pretreated with the superoxide dismutase inhibitor diethyldithiocarbamate. Cotreatment of this preparation with the cell-permeant O2− scavenger Tiron greatly reduced the deposition of blue formazan, and essentially no staining was found (Fig. 1B). Furthermore, mechanical removal of the endothelium did not result in staining of the medial smooth muscle (not shown), therefore precluding the endothelium as an artifactual barrier to nitroblue tetrazolium.
Figure 1.
Localization of O2−-generating activity in rabbit aorta. Cross-sections of a rabbit aorta were treated with the superoxide dismutase inhibitor diethyldithiocarbamate (10 mM, A) or diethyldithiocarbamate with Tiron (10 mM, B) for 30 min, followed by 90 min with nitroblue tetrazolium (100 μM). The aortic rings were then fixed, imbedded in paraffin, and photographed. Distribution of blue formazan deposits indicates the primary source of aortic O2− is the adventitia. Magnification, 28×.
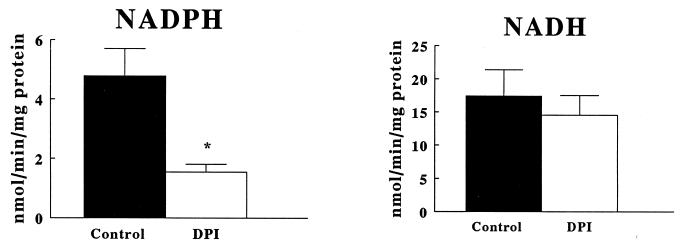
Table 1 shows the subcellular fractionation of O2−-generating activity in fibroblast homogenates. The fraction containing the most activity (the 28,000 × g pellet particulate fraction) also contained the greatest 5′ nucleotidase activity, consistent with the presence of plasma membranes. The pellet from a lower centrifugation to bring down cellular debris did not contain substantial activity, nor was there substantial relative activity in the soluble supernatant of the 28,000 × g centrifugation. As shown in Fig. 2, NADH-dependent O2−-generating activity was higher than NADPH-dependent activity yet was not significantly inhibitable by diphenylene iodonium (DPI, 100 μM). In contrast, the NADPH oxidase activity was significantly inhibited by DPI, a potent and highly selective flavoprotein inhibitor (Fig. 2). Shown in Table 2, neither of these activities was significantly inhibited by the cyclooxygenase inhibitor indomethacin (10 μM); the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME) (300 μM); the xanthine oxidase inhibitor oxypurinol (300 μM); or the NADH dehydrogenase inhibitor rotenone (100 μM).
Figure 2.
O2− generation by particulate fractions of quiescent aortic P3 fibroblasts. Cells were processed to obtain particulate fractions. Lucigenin chemiluminescence was measured, and units of chemiluminescence were converted to nmoles of O2− by standardizations with the xanthine oxidase–cytochrome c assay (15). Data are expressed as the mean nmoles of O2− generated per min per mg protein ±SEM. *Significance by a paired Student’s t test at P < 0.05.
Table 2.
O2−-generating activity of particulate fractions in aortic adventitial fibroblasts treated or not treated with various enzyme inhibitors
| Treatment | NADPH | NADH |
|---|---|---|
| Control | 1.9 ± 0.4 | 6.2 ± 1.8 |
| Indomethacin | 2.0 ± 0.5 | 6.0 ± 1.3 |
| l-NAME | 1.7 ± 0.2 | 7.7 ± 1.7 |
| Oxypurinol | 3.0 ± 0.8 | 4.8 ± 1.0 |
| Rotenone | 2.1 ± 0.4 | 6.5 ± 2.1 |
NADPH and NADH-dependent O2−-generating activity is expressed as nmol/min per mg of protein either untreated or treated with indicated inhibitors for 15 min prior to measurement. Data are means ± SEM. Values for control and inhibitor-treated samples were compared by a Student’s t test for paired analysis within columns. These data are representative of five to seven experiments. There were no significant differences found.
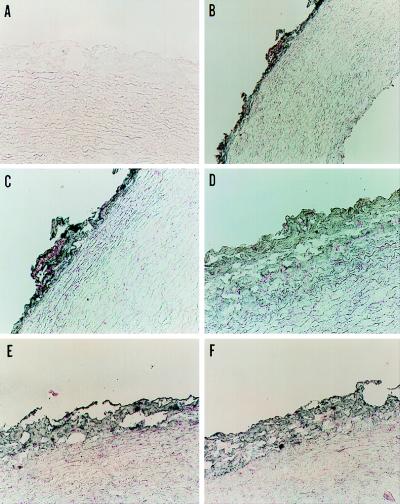
Immunohistochemistry of frozen sections of rabbit aorta revealed specific labeling of the adventitia with monoclonal antibodies against human phagocyte p22phox and gp91phox and recombinant p47phox and p67phox, whereas no staining was observed in other areas of the aorta (Fig. 3). As a control, we confirmed that these antibodies specifically recognized the appropriate NADPH oxidase protein in rabbit neutrophil lysates (data not shown). In addition to control sections stained with preimmune mouse serum (Fig. 3A), immunohistochemistry was also performed with both IgG 2a and IgG 1 antibody isotype controls, and neither showed cross-reactivity (not shown).
Figure 3.
Localization of NADPH oxidase proteins in rabbit aorta. Aortic sections were incubated with control nonimmune mouse serum (A) or monoclonal antibodies against p22phox (1:100) (B and C), gp91phox (1:200) (D), p47phox (1:20) (E), and p67phox (1:20) (F) at the dilutions shown, followed by gold-conjugated goat anti-mouse antibody. The sections were developed as described in Methods. Magnification is 190× (A and C–F) and 95× (B). The sections shown represent at least three stained sections for each antibody.
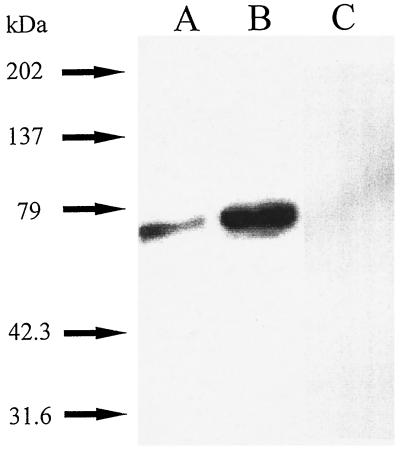
As shown in Fig. 4A, immunoblotting of samples of quiescent, fibroblast particulate fractions with a monoclonal antibody to p67phox (p67) confirmed the presence of p67phox. A Western blot done in parallel carried out with control antibody of the same isotype (IgG 2a with kappa light chain) at a higher concentration (1:500) did not show cross-reactivity (not shown). Moreover, preincubation of the p67phox polyclonal antibody with recombinant p67phox (23) completely prevented the appearance of cross-reactivity at approximately 67 kDa (Fig. 4C). To directly confirm the presence of phagocyte-like NADPH oxidase activity in rabbit aortic fibroblasts, we used this anti-p67phox monoclonal antibody to immunodeplete samples of p67phox. Sepharose beads were capable of sedimenting NADPH oxidase activity coupled with anti-p67phox (−54 ± 18%, n = 4, P < 0.05) from fibroblast particulate fractions compared with Sepharose beads coupled with control isotype (IgG 2a with kappa light chain) antibody. To confirm p67phox was the essential factor removed by the column, we added recombinant p67phox (22) to the flow-through fraction and were able to significantly increase activity 41 ± 6.7% (P < 0.05, n = 4, flow-through control versus flow-through to which recombinant 0.6 μg p67phox was added). We have tested and found that recombinant p67phox does not cause O2− generation on its own or when added to control particulates (n = 2).
Figure 4.
Immunoblotting of p67phox in particulate fractions of aortic adventitial fibroblasts. Fibroblast particulate fractions were separated by SDS-PAGE, transferred to PVDF, Western blotted with a 1:1000 dilution of monoclonal antibody to p67phox (22, 23), and developed as described. (A) 10 μg of fibroblast particulate fractions. (B) 10 μg of human neutrophil cytosol as a control. (C) 10 μg of fibroblast particulate fractions in which case 1:1000 p67phox anti-peptide antibody was preincubated with a 1.8 molar excess of recombinant p67phox (rec. p67). The anti-peptide antibody cross-reacted with a band at the identical molecular weight as did the monoclonal antibody (not shown). The electrophoretic mobility of the samples were compared with the mobility of prestained standard proteins. A representative of five experiments is shown.
Fig. 5 shows that treatment of adventitial fibroblasts with up to 10 nM AngII resulted in a concentration-dependent increase in NADPH/NADH-dependent O2− production in particulate fractions from these cells, whereas higher concentration (100 nM) had no stimulatory effect. DPI inhibited the maximal response (10 nM AngII) of NADH- and NADPH-dependent activity by 32 ± 8.3% and 80 ± 6.7%, respectively (P < 0.01 by paired t test for both NADH- and NADPH-dependent activity versus 10 nM AngII control in the absence of DPI). If one is to consider the relative amounts of DPI-inhibitable NADPH versus NADH-dependent O2− generation after treatment with 10 nM AngII, this represents 14.8 versus 12.1 nmol of O2− min/mg protein, respectively. We have examined whether AngII can induce p67phox expression and found no discernible change in signal on a Western blot using monoclonal and polyclonal antibodies (n = 3, not shown).
Figure 5.
AngII-induced O2− generation by particulate fractions of P3 rabbit aortic fibroblasts. Cells were either not treated or treated with AngII (A) in the presence or absence of 10 mM antagonists (B) for 3 h. Cells were processed as described to obtain particulate fractions. Lucigenin chemiluminescence was measured, and units of chemiluminescence were converted to nmoles of O2− by standardizations with the xanthine oxidase–cytochrome c assay. Data are expressed as the mean nmoles of O2− generated per min per mg protein ± SEM (n = 4–8 cell preparations). *Significance by a paired t test at P < 0.05.
The induction of ROS production by AngII in aortic fibroblasts peaked around 10 nM (Fig. 5A). Neither the type 1 receptor antagonist (Losartan, 10 μM) nor the type 2 receptor antagonist (PD123319, 10 μM) reduced the fibroblast response (Fig. 5B). However, the general AngII antagonist [Sar1,Thr8]AngII (10 μM) reversed the stimulation to basal levels.
DISCUSSION
The present studies demonstrate the site-specific localization of a constitutive phagocyte-like NADPH oxidase in the adventitia of the rabbit aorta. Histochemical staining of a cross-section of aorta suggested the specific localization of O2− production to the adventitia. The localization of NADPH oxidase protein to the aortic adventitia was confirmed by immunohistochemical staining, immunoblotting of fibroblast particulate fractions, and the immunodepletion of one of the essential NADPH oxidase components, p67phox. Characterization of particulate fractions of fibroblasts cultured from aortic adventitia demonstrates constitutive phagocyte-like NADPH oxidase activity that can be enhanced by AngII. Adventitial O2− derived from NADPH oxidase interferes with nitric oxide-induced relaxation (17) and is likely to be involved in the aberrant relaxations arising with hypertension (30, 31).
Characterization of the O2−-generating activity of fibroblast particulate fractions revealed higher NADH versus NADPH-dependent activity. In contrast, the NADPH oxidase activity was significantly inhibited by DPI, a potent and selective flavoprotein inhibitor, indicating an apparently more selective NADPH-dependent process similar to that found in phagocytic cells. Thus, there are at least two forms of O2−-generating enzymes in these cells. The negative results with O2− source inhibitors other than DPI preclude the likelihood of involvement of those other enzymes in the basal production of O2− in particulate fraction of these fibroblasts. Similar results were obtained in the intact aorta (15) and in rat vascular smooth muscle cells (11).
The presence of DPI-inhibitable NADPH oxidase activity in aortic adventitial fibroblasts suggested the possibility that this system was similar to that found in phagocytic cells. However, unlike in neutrophils, the fibroblast system is constitutively active (Fig. 2), and treatment of the cells with phorbol myristate acetate (1 μM, n = 2) for 10 min did not activate the oxidase above constitutive levels, at least minimally indicating that the mechanism of activation is different between neutrophils and fibroblasts (21).
To confirm the localization of a phagocyte-like NADPH oxidase in rabbit aortic adventitia, we analyzed frozen sections of rabbit aorta using immunohistochemistry with monoclonal antibodies against human phagocyte p22phox and gp91phox and recombinant p47phox and p67phox (22, 23). The adventitia in these preparations, however, were significantly less extensive than in the preparations for Fig. 1. This difference in the magnitude of adventitia is likely related to differential isolation methods but may also be related to the different fixing technique, which can cause compacting of tissue. In any event, these results again confirm the presence of a phagocyte-like NADPH oxidase system specifically localized to the rabbit aortic adventitia.
Immunoblotting of fibroblast particulate fractions confirmed the presence of p67phox in aortic adventitia. The estimated amount of p67phox in P1 aortic fibroblasts versus human neutrophils is approximately 80% based on densitometry normalized to protein amounts. Moreover, immunodepletion of p67phox caused a marked inhibition in NADPH oxidase activity, which was restored either by the addition of high-salt eluates or recombinant p67phox. Only partial restoration of activity with recombinant p67phox can be expected due to the known instability of p67phox and the NADPH oxidase in general (24, 25). Thus, the presence of p67phox demonstrated by Western blotting, along with our biochemical evidence for an NADPH oxidase activity in adventitial fibroblasts and its absolute dependence upon the presence of p67phox, provides compelling evidence that adventitial fibroblasts contain a phagocyte-like NADPH oxidase system.
AngII is a potent vasoconstrictor whose actions include direct contraction of smooth muscle through type 1 receptors (26, 27) and fibrosis of aorta and cardiac muscle due partially to the suppression of nitric oxide synthase activity (28, 29). Previously, Griendling et al. (11) showed that AngII stimulates NAD(P)H oxidase O2−-generating activity in smooth muscle of the rat aorta and that p22phox mRNA is present and involved in the expression of O2−-generating activity (16). Subsequent studies showed enhanced O2− during AngII-induced hypertension and confirmed the role of O2− in the maintenance of AngII-induced hypertension (30, 31). We were interested in determining if AngII could stimulate O2− production by NAD(P)H oxidase in aortic adventitial fibroblasts, the major cell type found in the vascular site of greatest production of O2− (Fig. 1 and ref. 15). The fact that DPI inhibited AngII-induced, NADH-dependent O2− generation, whereas basal NADH-dependent activity was not inhibited, suggests that a flavoprotein-dependent NADH oxidase may also be induced by AngII.
The induction of ROS production by AngII revealed species differences between rabbit aortic fibroblasts in this study and rat smooth muscle cells. Whereas rat aortic smooth muscle cells seem to respond concentration-dependently to AngII up to 1 μM (11), rabbit aortic fibroblasts seemed to reach a plateau in response at approximately 10 nM. Moreover, the type 1 AngII receptor antagonist, losartan, did not inhibit rises in NAD(P)H oxidase activity in response to AngII contrary to an inhibitory effect of losartan in rat smooth muscle cell preparations. As a control, we can show that the same preparations of AngII and losartan can increase and inhibit, respectively, the rise in calcium in rabbit aortic smooth muscle cells (data not shown). Recent findings indicate the presence of aminopeptidases in fibroblasts, which can convert AngII to active metabolites (32, 33). Perhaps one of those metabolites [i.e., Ang-(1–7), AngIII, or AngIV] may act through distinct receptors to induce NAD(P)H oxidase activity. Studies are underway to elucidate the precise agonist responsible for the increases in ROS production. In any case, the different effect of losartan in inhibiting the response in smooth muscle cells (11) compared with fibroblasts suggests that different receptors are involved in the two cell types or that this is a species difference between rat and rabbit.
Many vascular (34, 35) and nonvascular (36, 37) fibroses are influenced or mediated by O2− or one of its metabolites. Until recently, it was maintained that ambient O2− was a byproduct of mitochondrial respiration (38) or auto-oxidation of glucose (39). However, recently a number of reports have suggested a more substantial extra-mitochondrial enzymatic production of O2− in vascular and nonvascular tissue (8–12, 15). This production of O2− is lower in its apparent Vmax but is constitutively active and is expected to produce substantial amounts of O2− and perhaps more over the long-term. As our current studies indicate, O2− production in intact aorta is predominantly produced by adventitia, most likely by the fibroblasts that are the major cell type. Such O2− may modulate the growth and proliferation of these fibroblasts, as has been suggested for O2− (34, 35, 40). Indeed, stimulation of fibroblast growth coincides with matrix biosynthesis (41), and ROS derived from O2− have been implicated in the intimal proliferation response to arterial injury, characteristic of atherosclerosis (34). The role of O2− in the aberrant endothelium-dependent (nitric oxide) function of blood vessels from atherosclerotic/hypercholesterolemic animals has been shown convincingly in numerous studies (42–45) and may be predictive of chronic atherosclerosis and thrombosis. The role for O2− in both of these vascular processes in the etiology of atherosclerosis, for one, impels investigation of the involvement of this NADPH oxidase–O2− generation in the long-term fibrotic development of the disease.
The physiologic significance of these findings is further supported by recent work demonstrating that the action of nitric oxide released from nitrergic nerves is inhibited by endogenous O2− (46, 47). Because nitric oxide is released by nitrergic nerves into the adventitia of vascular tissue, it will be important to elucidate the role of this fibroblast NADPH oxidase–O2− generation on the fate of NO neurotransmission.
It had been suggested (48) that the adventitia acts as a physical barrier for nitric oxide; however, the nature of this impediment was not revealed. Our most recent work indicates that in vitro action of applied NO is impeded by adventitial O2−, and this impediment is derived from an NADPH oxidase in the rat aorta (17). In fact, the major site of O2− production in the rat aorta was determined to be the adventitia. Importantly, others have shown that rat aortic O2− levels derived from NAD(P)H oxidase increase with AngII-dependent forms of hypertension (30, 31). In general, this enzyme system seems to produce O2− levels that diminish the normal relaxant effect of nitric oxide on smooth muscle and is suggested to result in hypertension (30, 31). Understanding of the regulation of this adventitial, fibroblast source of O2− is important in understanding the regulation of bioactivity of the omnipresent nitric oxide and may be important in elucidating the mechanisms involved in the acute and chronic pathogenesis of hypertension.
Acknowledgments
We thank Drs. John F. Keaney and Joseph Loscalzo (Boston University) for valuable criticisms of this work; Robert Weisbrod (Boston University) and Laura Nelson (Montana State University) for technical assistance. We also thank Yue Du (Boston University) and Andy Blixt (Montana State University) for help in photographing stained tissue sections. This work was supported in part by NIH R29 Grant HL55425–02 and National American Heart Grant-in-Aid 95011900 (P.J.P); NIH RO1 (AR42426), USDA (NRICGP9502274), and an Arthritis Foundation Biomedical Science grant (M.T.Q). M.T.Q. is an Established Investigator of the American Heart Association.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ROS, reactive oxygen species; DPI, diphenylene iodonium; Ang, angiotensin.
References
- 1.Miller R A, Brittigan B E. J Invest Med. 1995;43:39–49. [PubMed] [Google Scholar]
- 2.Irani K, Xia Y, Zweier J L, Sollott S J, Der C J, Fearon E R, Sundaresan M, Finkel T, Goldschmidt-Clermont P. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 3.Coxon A, Rieu P, Barkalow F J, Askari S, Sharpe A H, Von Andrian U H, Arnaout M A, Mayadas T N. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 4.DeLeo F R, Quinn M T. J Leukocyte Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 5.Zhan S, Vazquez N, Zhan S, Wientjes F B, Budarf M L, Schrock E, Ried T, Green E D, Chanock S J. Blood. 1996;88:2714–2721. [PubMed] [Google Scholar]
- 6.Henderson L M, Chappell J B. Biochim Biophys Acta. 1996;1273:87–107. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 7.Knaus U G, Heyworth P G, Evans T, Curnutte J T, Bokoch G M. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 8.Acker H, Dufau E, Huber J, Sylvester D. FEBS Lett. 1989;256:75–78. doi: 10.1016/0014-5793(89)81721-1. [DOI] [PubMed] [Google Scholar]
- 9.Radeke H H, Cross A R, Hancock J T, Jones O T G, Nakamura M, Kaever V, Resch K. J Biol Chem. 1991;266:21025–21029. [PubMed] [Google Scholar]
- 10.Mohazzab K M, Wolin M S. Am J Physiol. 1994;267:L815–L822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- 11.Griendling K K, Minieri C A, Ollerenshaw J D, Alexander R W. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 12.Mohazzab K M, Kaminski P M, Wolin M S. Am J Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- 13.Jones S A, O’Donnell V B, Wood J D, Broughton J D, Hughes E J, Jones O T G. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 14.Meier B, Jesaitis A J, Emmendorfer A, Roesler J, Quinn M T. Biochem J. 1991;289:481–486. doi: 10.1042/bj2890481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagano P J, Ito Y, Tornheim K, Gallop P M, Tauber A I, Cohen R A. Am J Physiol. 1995;268:H2274–H2280. doi: 10.1152/ajpheart.1995.268.6.H2274. [DOI] [PubMed] [Google Scholar]
- 16.Ushio-Fukai M, Zafari A M, Fukui T, Ishizaka N, Griendling K K. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Pagano P J, Du Y, Cayatte A J, Brecher P, Cohen R A. FASEB J. 1997;11:A477. [Google Scholar]
- 18.Masur S K, Dewal H S, Dinh T T, Erenburg I, Petridou S. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farivar R S, Chobanian A V, Brecher P. Circ Res. 1996;78:759–769. doi: 10.1161/01.res.78.5.759. [DOI] [PubMed] [Google Scholar]
- 20.Robertson A K, Cross A R, Jones O T G, Andrew P W. J Immunol Methods. 1990;133:175–179. doi: 10.1016/0022-1759(90)90357-2. [DOI] [PubMed] [Google Scholar]
- 21.Opdahl H, Benestad H B, Nicolaysen G. Acta Anaesthesiol Scand. 1987;31:491–498. doi: 10.1111/j.1399-6576.1987.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 22.Burritt J B, Quinn M T, Jutila M A, Bond C W, Jesaitis A J. J Biol Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- 23.DeLeo F R, Ulman K V, Davis A R, Jutila K L, Quinn M T. J Biol Chem. 1996;271:17013–17020. doi: 10.1074/jbc.271.29.17013. [DOI] [PubMed] [Google Scholar]
- 24.Erickson R W, Malawista S E, Garrett M C, Van Blaricom G, Leto T L, Curnutte J T. J Clin Invest. 1992;89:1587–1595. doi: 10.1172/JCI115753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura M, Takeshita M, Curnutte J T, Uhlinger D J, Lambeth J D. J Biol Chem. 1992;267:7529–7538. [PubMed] [Google Scholar]
- 26.Iyer S N, Lu D, Katovich M J, Raizada M K. Proc Natl Acad Sci USA. 1996;93:9960–9965. doi: 10.1073/pnas.93.18.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitami Y, Okura T, Marumoto K, Wakamiya R, Hiwada K. Biochem Biophys Res Commun. 1992;188:446–452. doi: 10.1016/0006-291x(92)92405-m. [DOI] [PubMed] [Google Scholar]
- 28.Kato H, Hou J, Chobanian A V, Brecher P. Hypertension. 1996;28:153–158. doi: 10.1161/01.hyp.28.2.153. [DOI] [PubMed] [Google Scholar]
- 29.Hou J, Kato H, Cohen R A, Chobanian A V, Brecher P. J Clin Invest. 1995;96:2469–2477. doi: 10.1172/JCI118305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman B A, Griendling K K, Harrison D G. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laursen J B, Rajagopalan S, Galis Z, Tarpey M, Freeman B A, Harrison D G. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 32.Vaghy P L, Russell J S, Lantry L E, Stephens R E, Ward P E. Peptides. 1995;16:1367–1373. doi: 10.1016/0196-9781(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 33.Piela-Smith T H, Korn J H. Cell Immunol. 1995;162:42–48. doi: 10.1006/cimm.1995.1049. [DOI] [PubMed] [Google Scholar]
- 34.Rao R N, Berk B C. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 35.Rao G N, Lasségue B, Griendling K K, Alexander R W, Berk B C. Nucleic Acids Res. 1993;21:1259–1263. doi: 10.1093/nar/21.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilinc C, Ozcan O, Karaoz E, Sunguroglu K, Kutluay T, Karaca L. J Basic Clin Physiol Pharmacol. 1993;4:249–269. doi: 10.1515/jbcpp.1993.4.3.249. [DOI] [PubMed] [Google Scholar]
- 37.Denis M. Inflammation. 1995;19:207–219. doi: 10.1007/BF01534462. [DOI] [PubMed] [Google Scholar]
- 38.Pitkänen S, Robinson B H. J Clin Invest. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamura M, Heinecke J W, Chait A. J Clin Invest. 1994;94:771–778. doi: 10.1172/JCI117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murrell G A, Francis M J, Bromley L. Biochem J. 1990;265:659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahari V M, Vuorio T, Nanto-Salonen K, Vuorio E. Biochim Biophys Acta. 1984;78:183–186. doi: 10.1016/0167-4781(84)90136-2. [DOI] [PubMed] [Google Scholar]
- 42.Mügge A, Elwell J H, Peterson T E, Harrison D G. Am J Physiol. 1991;260:C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- 43.Keaney J F, Jr, Xu A, Cunningham D, Jackson T, Frei B, Vita J A. J Clin Invest. 1995;95:2520–2529. doi: 10.1172/JCI117953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keaney J F, Jr, Gaziano J M, Xu A, Frei B, Curran-Celentano J, Shwaery G T, Loscalzo J, Vita J A. J Clin Invest. 1994;93:844–851. doi: 10.1172/JCI117039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keaney J F, Jr, Gaziano J M, Xu A, Frei B, Curran-Celentano J, Shwaery G T, Loscalzo J, Vita J A. Proc Natl Acad Sci USA. 1993;90:11880–11884. doi: 10.1073/pnas.90.24.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lilley E, Gibson A. Br J Pharmacol. 1995;116:3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C G, Rand M J. Br J Pharmacol. 1996;118:57–62. doi: 10.1111/j.1476-5381.1996.tb15366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinhorn R H, Morin F C, Russell J S. J Clin Invest. 1994;94:1883–1888. doi: 10.1172/JCI117538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hacker G W, Zehbe I, Muss W H, Hauser-Kronberger C, Graf A-H, Dietze O. CellVision. 1995;2:247–253. [Google Scholar]
- 50.Liochev S I, Fridovich I. Arch Biochem Biophys. 1997;337:115–120. doi: 10.1006/abbi.1997.9766. [DOI] [PubMed] [Google Scholar]