Abstract
Engagement of the mast cell high-affinity receptor for immunoglobulin E (IgE), FcɛRI, induces tyrosine phosphorylation of Syk, a non-receptor tyrosine kinase, that has been demonstrated as critical for degranulation. Herein we describe a synthetic compound, ER-27319, as a potent and selective inhibitor of antigen or anti-IgE-mediated degranulation of rodent and human mast cells. ER-27319 affected neither Lyn kinase activity nor the antigen-induced phosphorylation of the FcɛRI but did effectively inhibit the tyrosine phosphorylation of Syk and thus its activity. As a consequence, tyrosine phosphorylation of phospholipase C-γ1, generation of inositol phosphates, release of arachidonic acid, and secretion of histamine and tumor necrosis factor α were also inhibited. ER-27319 did not inhibit the anti-CD3-induced tyrosine phosphorylation of phospholipase C-γ1 in Jurkat T cells, demonstrating a specificity for Syk-induced signals. In contrast the tyrosine phosphorylation and activation of Syk, induced by in vitro incubation with the phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) of FcɛRI γ subunit or by antigen activation of RBL-2H3 cells, was specifically inhibited by ER-27319. However, when ER-27319 was added to immunoprecipitated Syk, derived from activated cells, no effect was seen on Syk activity. ER-27319 did not inhibit the tyrosine phosphorylation of Syk induced by activation in the presence of Igβ ITAM or the anti-IgM-induced phosphorylation of Syk in human peripheral B cells. Therefore, ER-27319 selectively interferes with the FcɛRI γ phospho-ITAM activation of Syk in vitro and in intact cells. These results confirm the importance of Syk in FcɛRI-mediated responses in mast cells and demonstrate the mast cell selectivity and therapeutic potential of ER-27319 in the treatment of allergic disease.
Keywords: immunoreceptor tyrosine-based activation motif, secretion
Allergen-induced activation of the high-affinity receptor for IgE (FcɛRI) in mast cells and basophils plays a pivotal role in the initiation of allergic and inflammatory reactions. As a result of receptor engagement, mast cells and basophils release a variety of inflammatory mediators, such as histamine, arachidonic acid metabolites, and cytokines (1, 2). The activation of protein tyrosine kinases (PTKs) is one of the early and critical signaling events following FcɛRI engagement (3). Since FcɛRI belongs to the family of multisubunit antigen receptors that do not possess intrinsic PTK activity in their structure (1), the recruitment and activation of non-receptor PTKs is crucial for FcɛRI-mediated signal transduction (4).
FcɛRI is a tetrameric receptor composed of an α subunit, a β subunit, and a homodimer of disulfide-linked γ subunits (5). The cytoplasmic domains of β and γ subunits contain sequences known as the immunoreceptor tyrosine-based activation motifs (ITAMs) (6, 7). The phosphorylation of the tyrosines of the ITAM allows the interaction of ITAM-containing receptors with signaling proteins that have phosphotyrosine-recognizing Src homology 2 (SH2) domains (8, 9).
Syk kinase is activated in FcɛRI-mediated signaling in mast cells (10, 11). Syk possesses two SH2 domains toward the amino terminus of the protein that mediate high-affinity interactions with the tyrosine-phosphorylated ITAM of the β and γ subunits of FcɛRI (9, 12). The association of Syk with the ITAM of the γ subunit induces the phosphorylation and activation of Syk (11–13) and stimulates subsequent signaling events, including activation of phospholipase C-γ1 (PLC-γ1) and protein kinase C (PKC). Thus it is possible that selective inhibitors of Syk or specific blockers of the association of Syk with ITAMs may prevent signaling by FcɛRI.
Here, we report that a synthetic acridone-related compound, ER-27319, inhibits mast cell responses by inhibiting the phosphorylation and activation of Syk in these cells.
MATERIALS AND METHODS
Reagents and Animals.
Minimal essential medium (MEM), macrophage-SFM medium, and Dulbecco’s phosphate-buffered saline (D-PBS) were from GIBCO/BRL. 5-Hydroxy[2-14C]tryptamine binoxalate, 5-hydroxy[1,2-3H(N)]tryptamine binoxalate, [1-14C]arachidonic acid, and myo-[2-3H(N)]inositol were obtained from DuPont NEN. Histamine EIA (enzyme immunoassay) Kit was from Immunotech (Marseille, France). Peptide-leukotriene (pLT) EIA Kit and prostaglandin D2-MOX EIA Kit were from Cayman Chemicals (Ann Arbor, MI). Mouse tumor necrosis factor α (TNFα) EIA Kit was obtained from Genzyme. Recombinant human stem cell factor (SCF) was from PeproTech (Rocky Hill, NJ). Mouse anti-human mast cell tryptase antibody and anti-human mast cell chymase antibody were from Chemicon. Antibody to phosphotyrosine (PY20) coupled to horseradish peroxidase was from ICN Immunologicals (Cleveland, OH). Agarose-conjugated PY20 and a mouse monoclonal antibody to protein kinase C δ were from Transduction Laboratories (Lexington, KY). Antibodies to PLC-γ1 and Lyn were from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal antibody to Syk was prepared by the method of Minoguchi et al. (13). Goat antibody to glutathione S-transferase (GST) was from Pharmacia. o-Dinitrophenyl (DNP)25-BSA and DNP-specific monoclonal mouse IgE were kindly supplied by Henry Metzger (National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD). Purified GST-fusion protein encoding the phosphorylation site of HS1, targeted by Syk kinase, was kindly provided by Ulrich Blank (Immuno-Allergie, Institut Pasteur, Paris). Baculovirus expressed recombinant PKCδ was kindly provided by Peter M. Blumberg (National Cancer Institute, National Institutes of Health, Bethesda, MD). Phosphorylated ITAMs of the γ subunit of the FcɛRI, DGVpYTGLSTRNQETpYETLK, and the β subunit of the B cell antigen receptor (Igβ), DHTpYEGLNIDQTATpYEDIVT, were synthesized by the Peptide Institute (Osaka, Japan). ER-27319 [3,4-dimethyl-10(-3-aminopropyl)-9-acridone oxalate] and Ro31–8425 were synthesized in our laboratory. Enhanced chemiluminescence (ECL) system was from Amersham International (Buckinghamshire, England). Male Sprague–Dawley rats were purchased from Japan SLC (Hamamatsu, Japan).
Stock solutions of the pharmacological agents Ro31–8425, phorbol 12-myristate 13-acetate (PMA), calcium ionophore A23187, genistein, herbimycin A, and ER-27319 were prepared in dimethyl sulfoxide. They were diluted directly in buffer to give a final dimethyl sulfoxide concentration of <0.1% (vol/vol). Controls were also incubated with an equal concentration of carrier solvent.
Preparation of Cells.
RBL-2H3 cells were incubated overnight with DNP-specific IgE, 0.5 μg/ml; myo-[3H]inositol, 4 μCi/ml (1 μCi = 37 kBq); [14C]arachidonic acid, 1 μCi/ml; and 5-hydroxy[14C]tryptamine or 5-hydroxy[3H]tryptamine, 1 μCi/ml, as described (14). Cultures (2 × 105 cells in 0.4 ml) were incubated in complete growth medium (minimal essential medium with Earle’s salts and 15% heat-inactivated fetal calf serum) in 24-well culture plates. All cultures were washed twice in the desired buffer solutions before the final addition of 0.2 ml of fresh buffer and stimulants.
Rat peritoneal mast cells were obtained as described (15). Cultured human mast cells were obtained by the method of Saito et al. (16). Differentiation of the cultured human mast cells was monitored periodically with mouse antibody to tryptase and chymase. The human mast cells obtained were incubated overnight with human IgE, 10 μg/ml; and [14C]arachidonic acid, 1 μCi/ml, to sensitize the cells. Cells (1 × 106 in 0.5 ml) were stimulated with anti-human IgE antibody. Human B cells were partially purified from peripheral blood mononuclear cells of healthy adult donors by depletion of CD3+ cells with a magnetic cell sorter (MACS, Miltenyi Biotec, Bergish Gladbach, Germany).
Measurements of Mediator Release.
The accumulation of total [3H]inositol phosphates was determined as previously described (17). The release of radiolabeled arachidonic acid and 5-hydroxytryptamine was also determined as described (18). The release of histamine was analyzed by enzyme immunoassay according to the manufacturer’s instructions. Measurements of pLTs, prostaglandins, and TNFα were performed by enzyme immunoassays according to the manufacturer’s instructions.
Analysis of Protein Tyrosine Phosphorylation and Western Blots.
For tyrosine phosphorylation of total cellular proteins, cells were lysed by the addition of ice-cold lysis buffer [10 mM phosphate buffer (pH 7.5) containing 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 50 mM NaCl, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 50 μg/ml leupeptin, 10 units/ml aprotinin, and 0.1% NaN3]. Samples were centrifuged (14,000 × g for 5 min at 4°C), mixed with SDS sample buffer [0.125 M Tris⋅HCl, pH 6.8/4% (wt/vol) SDS], boiled for 3 min, and resolved by SDS/PAGE. For immunoprecipitation experiments with antibody to Syk, cells were lysed by ice-cold lysis buffer [20 mM Hepes buffer (pH 7.2) containing 1% Triton X-100, 10% (vol/vol) glycerol, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 50 μg/ml leupeptin, and 10 units/ml aprotinin]. Cell lysates were incubated with the antibody at 4°C for 3 hr, and immunoprecipitated proteins were recovered. Immunoprecipitation of PLC-γ1 was performed exactly as described in a previous study (14). Samples were resolved by SDS/PAGE.
Immunoprecipitation of FcɛRI by means of cell-bound IgE was as described (19) with the exception that cells were incubated in the presence or absence of 30 or 60 μM ER-27319 for 10 min at 37°C before stimulation with DNP-BSA. For immunoprecipitation of FcɛRI by the γ subunit, 32 μg of an antibody (IgY fraction) raised in chickens to a synthetic peptide of the γ subunit of the FcɛRI and 100 μg of rabbit anti-chicken IgY (Jackson Immunoresearch) were added for 1 hr at 4°C. Subsequently, protein A-Sepharose was added overnight. The recovered proteins were processed as described (19).
Western blots were as described (19). Detection of resolved proteins with specific antibodies was as indicated in figure legends. Reactive proteins were visualized by ECL.
Measurement of Syk Tyrosine Phosphorylation Induced by Phosphorylated ITAMs or B Cell Stimulation.
To test the effect of ER-27319 on the tyrosine phosphorylation of Syk induced by phosphorylated ITAMs, intact RBL-2H3 cells were homogenized in a Dounce homogenizer (100–150 strokes) in the presence of ice-cold lysis buffer [20 mM Tris⋅HCl (pH 7.5) containing 150 mM NaCl, 10 mM KCl, 0.5 mM EGTA, 2 mM dithiothreitol, 5 mM NaF, 1 mM Na3VO4, 50 μg/ml leupeptin, 10 units/ml aprotinin, and 100 μg/ml soybean trypsin inhibitor]. Homogenates were centrifuged (100,500 × g for 1 hr at 4°C) to collect soluble proteins. Tyrosine phosphorylation of Syk in the presence of ITAMs was done as described by Shiue et al. (20).
We also tested the effect of ER-27319 on anti-IgM-induced Syk phosphorylation in human peripheral B cells. Human B cells were incubated with ER-27319 for 15 min at 37°C and then stimulated with anti-IgM (μ chain, Organon Teknika–Cappel) for various times. Syk was subsequently immunoprecipitated, resolved by SDS/PAGE, and analyzed by Western blotting.
Immune Complex in Vitro Kinase Assays.
Syk was immunoprecipitated from cells that were treated or not with ER-27319 (30 or 100 μM) or piceatannol (100 nM) for 10 min at 37°C followed by antigen activation for 3 or 10 min. The Syk immune complexes were incubated with 5 μg of GST-HS1 in kinase buffer as described by Shiue et al. (20) for 5 min at 30°C with constant mixing. In some experiments ER-27319 or piceatannol was added in the kinase buffer to Syk immune complexes isolated from activated cells prior to addition of GST-HS1 and ATP. For Lyn immune-complex assays, Lyn was immunoprecipitated from lysates of activated cells treated with herbimycin A (10 μM), genistein (300 μM), or ER-27319 (30 or 100 μM), and the activity was assayed as described (4). All reactions were quenched by the addition of 5-fold concentrated SDS sample buffer and boiling.
RESULTS
ER-27319 Inhibits Antigen-Induced Cell Responses in RBL-2H3 Cells.
Table 1 shows that ER-27319 can effectively inhibit the antigen-induced secretion of 5-hydroxytryptamine, generation of inositol phosphates, release of arachidonic acid, and TNFα production in a concentration-dependent manner, achieving a half-maximal inhibition (IC50) at a concentration of 10 μM. Almost complete inhibition of all measured responses (>80%) was achieved at a concentration of ER-27319 of 30 μM. Treatment of RBL-2H3 cells with ER-27319 before addition of the secretion-stimulating agents, A23187 and phorbol 12-myristate 13-acetate, did not significantly inhibit the secretion induced by these agents (Table 1). At concentrations of up to 30 μM only minimal inhibition of secretion was observed (13.8% ± 2.7%). In contrast, the PKC inhibitor, Ro31–8425, selectively inhibited only the secretory response without affecting other responses to antigen (data not shown).
Table 1.
Inhibition of RBL-2H3 cell responses by ER-27319
| ER-27319, μM | Inhibition, %
|
|||||
|---|---|---|---|---|---|---|
| DNP-BSA*
| ||||||
| TNFα
|
A23187 + PMA†
|
|||||
| 5-HT | IP | AA | In cells | In medium | 5-HT | |
| 3 | 21.2 ± 2.9 | 24.4 ± 1.5 | 28.6 ± 2.4 | 24.8 ± 3.5 | 29.7 ± 4.8 | 7.6 ± 2.8 |
| 10 | 46.7 ± 3.3 | 46.9 ± 2.1 | 48.5 ± 1.9 | 48.2 ± 1.3 | 47.8 ± 3.1 | 8.8 ± 0.8 |
| 30 | 83.8 ± 1.1 | 81.3 ± 0.7 | 84.8 ± 0.9 | 81.2 ± 1.6 | 83.5 ± 2.9 | 13.8 ± 2.7 |
Cells were incubated with the indicated concentrations of ER-27319 for 10 min before stimulation with antigen (DNP-BSA, 10 ng/ml) or A23187 and phorbol 12-myristate 13-acetate (PMA) (100 nM and 20M, respectively) for 15 min at 37°C. Cellular responses analyzed: 5-HT, 5-hydroxytryptamine; IP, inositol phosphates; AA, arachidonic acid; and TNFα. Values are presented as the percent inhibition of the response in the absence of ER-27319. Values are the mean ± SE of three experiments.
Normal responses to DNP-BSA activation were 38.4% ± 1.2% (5-HT), 18.1% ± 1.1% (IP), 5.88% ± 1.1% (AA), 107.6 ± 9.9 pg per 106 cells (TNF in cells), and 308 ± 16.7 pg/ml (TNF in medium).
A23187 plus PMA induced 5-HT secretion 28.8% ± 3.2%.
Collectively, the data demonstrate that ER-27319 is likely to mediate its inhibitory effect upstream of PKC, because it inhibits both hydrolysis of phosphatidyinositol and metabolism of arachidonic acid. This promotes the idea that this compound may target an early step proximal to the activation of the FcɛRI
ER-27319 Inhibits Antigen-Induced Cell Responses in Rat Peritoneal and Human Cultured Mast Cells.
We studied the effect of ER-27319 on antigen-activated rat peritoneal mast cells. The detectable levels of histamine, pLTs, and prostaglandin D2 in these cells were 521.2 ± 12.1 ng per 105 cells, 55.9 ± 2.3 pg per 105 cells, and 8.6 ± 0.7 ng per 105 cells, respectively. ER-27319 blocked the release of these mediators in a dose-dependent manner (Table 2). The IC50 of ER-27319 in rat peritoneal mast cells was approximately 10 μM, essentially the same as that in RBL-2H3 cells. Furthermore, 30 μM ER-27319 also inhibited the measured responses >80%. On the basis of the results shown in Tables 1 and 2, treatment of mast cells with ER-27319 inhibits both early and late events in mast cell responses.
Table 2.
Inhibition of primary mast cell responses by ER-27319
| ER-27319, μM | Inhibition, %
|
||||
|---|---|---|---|---|---|
| Rat peritoneal mast cells*
|
Human cultured mast cells†
|
||||
| Histamine | pLTs | PGD | Histamine | AA | |
| 3 | 28.2 ± 3.7 | 15.5 ± 2.3 | 41.4 ± 1.9 | 29.7 ± 5.7 | 34.2 ± 4.4 |
| 10 | 38.3 ± 5.9 | 30.1 ± 6.2 | 47.7 ± 4.9 | 41.6 ± 5.1 | 49.3 ± 5.9 |
| 30 | 84.8 ± 3.1 | 90.2 ± 2.9 | 80.7 ± 3.9 | 89.2 ± 3.1 | 80.2 ± 2.9 |
Cells were incubated with the indicated concentrations of ER-27319 for 10 min before DNP-BSA (0.3 μg/ml) or anti-human IgE (1 μg/ml) stimulation for an additional 15 min at 37°C. Mast cell responses measured: histamine, histamine release; pLTs, peptide leukotrienes; PGD, prostaglandin D2; AA, arachidonic acid release. Values are the mean ± SE of three experiments.
Values of each of the responses to antigen in the absence of ER-27319 were 521.2 ± 12.1 ng per 105 cells (histamine), 55.9 ± 2.3 pg per 105 cells (pLTs), and 8.6 ± 0.7 ng per 105 cells (PGD).
Anti-human IgE induced histamine release 33.2% ± 7.9% (103.8 ± 28.2 ng per 105 cells) and arachidonic acid release 8.21% ± 0.91%.
Human mast cells were obtained by long-term (8–10 weeks) culture of umbilical cord blood mononuclear cells in the presence of stem cell factor (SCF), interleukin-6 (IL-6), and prostaglandin E2 (PGE2) (16). The cultured umbilical cord blood mononuclear cells were periodically analyzed for histamine content and tryptase-positive cells. After 8 weeks 90% of the cells were tryptase positive and had a histamine content of 498.9 ± 110.6 ng per 105 cells (data not shown), values consistent with previous reports (16). Treatment of the 8- to 10-week cultured human mast cells with human IgE followed by anti-human IgE stimulated histamine secretion (103.8 ± 28.2 ng per 105 cells) and arachidonic acid release (8.21% ± 0.91%). As shown in Table 2, ER-27319 effectively inhibited the anti-IgE-induced release of histamine and arachidonic acid (>80% at 30 μM). These results demonstrate that in cultured human cells that are functionally and immunophenotypically mature mast cells, ER-27319 serves as an effective inhibitor of degranulation and arachidonic acid release.
ER-27319 Inhibits the Tyrosine Phosphorylation of Syk in Mast Cells.
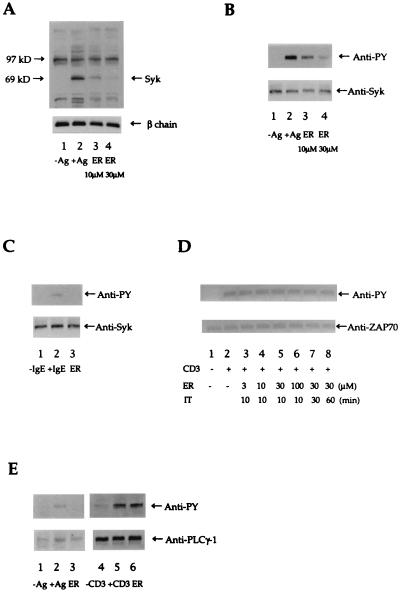
Fig. 1A shows that ER-27319 at 10 μM clearly inhibited the tyrosine phosphorylation of proteins with the apparent molecular mass of Syk, and 30 μM concentrations resulted in almost complete inhibition. Some inhibition of tyrosine phosphorylation of other proteins was observed. As shown in Fig. 1B, immunoprecipitation of Syk revealed that ER-27319 at 10 μM inhibited tyrosine phosphorylation of Syk. Furthermore, concentrations of ER-27319 of up to 30 μM proved more effective in the inhibition of Syk tyrosine phosphorylation. Relative quantitation of immunoblots by densitometric analysis showed that Syk phosphorylation was inhibited by 57% ± 9.2% and 87% ± 8.9% in the presence of 10 μM or 30 μM ER-27319, respectively. We also tested whether ER-27319 (30 μM) would inhibit the tyrosine phosphorylation of Syk in cultured human mast cells. Fig. 1C demonstrates that this compound could effectively inhibit the tyrosine phosphorylation of Syk in these cells. These results suggest that ER-27319 may preferentially target the induction of Syk tyrosine phosphorylation.
Figure 1.
(A) Effect of inhibitors on antigen-induced tyrosine phosphorylation of intracellular proteins in RBL-2H3 cells. Cells (1 × 106 cells per well in 12-well plates) were incubated with 10 μM or 30 μM ER-27319 (ER) for 10 min before stimulation with antigen (Ag; DNP-BSA, 10 ng/ml). Proteins were resolved and transferred to nitrocellulose membranes. The membranes were probed with antibody to phosphotyrosine (Upper) and then reprobed with antibody to FcɛRI β chain (Lower) to normalize for the protein content in each lane. The apparent migration of Syk or FcɛRI β chain is indicated. (B) Effect of ER-27319 on the tyrosine phosphorylation of Syk. Cells (1 × 106) were incubated with 10 μM or 30 μM ER-27319 for 10 min before stimulation with antigen. Cells were lysed and allowed to react with antibody to Syk. Immunoprecipitated proteins was resolved by SDS/10% PAGE and transferred to nitrocellulose membranes. Resolved proteins were probed with antibody to phosphotyrosine (Anti-PY), and subsequently membranes were reprobed with antibody to Syk (Anti-Syk). (C) Immunoprecipitation of Syk from human cultured mast cells. Experimental conditions were identical to those in B but only a concentration of 30 μM ER-27319 was used. (D) Effect of ER-27319 on tyrosine phosphorylation of ZAP-70 in anti-CD3-stimulated Jurkat cells. Cells (1 × 106) were incubated with ER-27319 (at indicated concentrations) for the indicated incubation time (IT) prior to activation with anti-CD3 for 10 min. Cells were lysed and ZAP-70 was immunoprecipitated, resolved, and analyzed by Western blotting with anti-PY and subsequently with anti-ZAP-70. (E) Effect of ER-27319 on tyrosine phosphorylation of PLC-γ1 in antigen-stimulated RBL-2H3 cells or in anti-CD3-stimulated Jurkat T cells. RBL-2H3 cells (4 × 107 cells per 150-cm2 dish) or Jurkat T cells (1 × 107 cells) were incubated with 30 μM ER-27319 (ER) for 10 min before stimulation with 10 ng/ml DNP-BSA (Ag) or 2 μg/ml anti-CD3 (CD3) for 10 min. PLC-γ1 was immunoprecipitated, resolved, and transferred to nitrocellulose membranes. Membranes were probed with anti-PY and subsequently reprobed with anti-PLC-γ1.
Therefore we asked whether ER-27319 was Syk selective or whether it might also inhibit the tyrosine phosphorylation of the Syk-related ZAP-70 kinase found in Jurkat T cells. Fig. 1D shows that ER-27319 at concentrations of up to 100 μM and preincubation times of up to 60 min did not inhibit the tyrosine phosphorylation of ZAP-70 in response to anti-CD3 stimulation of the Jurkat cells. Because PLC-γ1, a known target of Syk activity (21), is found in both the RBL-2H3 cells and Jurkat T cells, we postulated that if ER-27319 is Syk selective the tyrosine phosphorylation of PLC-γ1 in Jurkat cells should not be affected by this drug. Following a 10-min stimulation of RBL-2H3 cells with antigen or of Jurkat T cells with antibody to CD3, tyrosine phosphorylation of PLC-γ1 was inhibited by ER-27319 only in the RBL-2H3 cells (Fig. 1E).
Collectively, the results show that ER-27319 mediates its effect prior to activation of PLC-γ1. Since ER-27319 did not inhibit the ZAP-70-induced tyrosine phosphorylation of PLC-γ1 in Jurkat T cells, this suggests that the specific target of ER-27319 is Syk kinase.
ER-27319 Inhibits Neither Lyn Kinase Activity nor the Phosphorylation of the IgE Receptor β and γ Subunits.
We examined whether ER-27319 inhibits Syk phosphorylation by inhibiting Lyn kinase activity, which is required for phosphorylation of the β and γ subunits of FcɛRI and the subsequent binding and activation of Syk (10–13). As shown in Fig. 2A, the activity of Lyn isolated from cells treated with ER-27319 (30 μM) was not inhibited, as demonstrated by its ability to phosphorylate PKCδ (22). In fact, we consistently saw a small enhancement in Lyn activity when cells were treated with ER-27319 (Fig. 2A, lane 4). In contrast, pretreatment of the cells with herbimycin or genistein effectively inhibited Lyn activity (Fig. 2A, lanes 2 and 3). Furthermore, the addition of ER-27319 (100 μM) to Lyn immune complexes isolated from activated cells had no effect on Lyn activity (data not shown).
Figure 2.
(A) Effect of ER-27319 on Lyn kinase activity. IgE-sensitized RBL-2H3 cells were incubated for 10 min in the presence or absence of carrier solvent dimethyl sulfoxide (DMSO), 10 μM herbimycin, 300 μM genistein, or 30 μM ER-27319 as indicated. Lyn was immunoprecipitated (lanes 1–4) from cells lysed in β-octyl glucoside (60 mM), and a control immunoprecipitate with IgG of unknown specificity is shown in lane 5. In vitro kinase assays with immune complexes were carried out using recombinant PKCδ as a substrate. Proteins were resolved by SDS/PAGE and analyzed by Western blotting using an antibody to phosphotyrosine and subsequently antibodies to PKCδ and Lyn to normalize for protein content. One of two representative experiments is shown. (B) Effect of ER-27319 on antigen-induced tyrosine phosphorylation of the FcɛRI. IgE-sensitized RBL-2H3 cells were incubated with or without 30 μM ER27319 before stimulation with antigen as indicated. Lanes 1 and 2 were immunoprecipitated with goat IgG of unknown specificity; lane 3 was immunoprecipitated with preimmune chicken IgY; lanes 4 and 5 were immunoprecipitated with goat IgG specific to mouse IgE; lanes 6, 7, and 8 were immunoprecipitated with chicken IgY specific to FcɛRI γ subunit. The resolved proteins were probed with antibody to phosphotyrosine, and the apparent migration of FcɛRI β and γ subunits is indicated. Detection of γ subunit of FcɛRI was by incubation with a chicken antibody to γ chain. One representative of four experiments is shown.
As shown in Fig. 2B (lanes 4 and 5), immunoprecipitation of the FcɛRI, by means of cell surface-bound IgE, from cells treated or not with ER-27319, clearly demonstrated that tyrosine phosphorylation of the receptor subunits is also unaffected by this agent. Moreover, immunoprecipitation with an antibody to the FcɛRI γ subunit that does not react well with the phosphorylated species of this subunit (Fig. 2B, lanes 7 and 8) revealed that a limited recovery of activated receptor gave identical results. These latter conditions convincingly demonstrate that a possible inhibition of receptor phosphorylation by ER-27319 is not masked by phosphotyrosine signals beyond the linear range of detection. Further experiments with concentrations of ER-27319 of up to 60 μM did not inhibit receptor subunit phosphorylation (data not shown). The results demonstrate that the mechanism of ER-27319 action does not involve the inhibition of Lyn kinase activity and receptor phosphorylation.
ER-27319 Specifically Inhibits Tyrosine Phosphorylation of Syk and Its Activity When Induced by the FcɛRI.
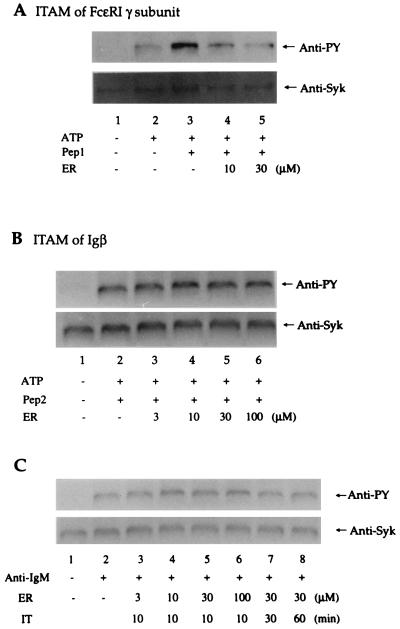
The tyrosine phosphorylation and activation of Syk by the phosphorylated γ ITAM of FcɛRI and the phosphorylated Igα/Igβ ITAMs of the B cell antigen receptor has been demonstrated (20, 23). Since ER-27319 inhibits Syk activation in RBL-2H3 cells, we tested whether ER-27319 might inhibit the tyrosine phosphorylation of Syk induced by the FcɛRI γ subunit ITAM in vitro. We also tested whether ER-27319 might inhibit the activation of Syk induced by the phosphorylated Igβ ITAM. Cytosolic lysates of RBL-2H3 cells, which contain most of the cellular Syk, were incubated with 50 μM ATP and phosphorylated ITAMs. Fig. 3 shows the tyrosine phosphorylation of Syk in response to incubations with the phosphorylated γ subunit or Igβ ITAMs. ER-27319 inhibited the tyrosine phosphorylation of Syk induced by the phospho-γ ITAM of the FcɛRI γ (Fig. 3A) but not the tyrosine phosphorylation of Syk induced by the phospho-Igβ ITAM (Fig. 3B). In the presence of 10 μM or 30 μM ER-27319, Syk phosphorylation induced by the phospho-γ ITAM of the FcɛRI γ was inhibited by 68% ± 9.9% and 93% ± 3.3%, respectively. However, concentrations of ER-27319 of up to 100 μM had no effect on the Igβ ITAM-induced phosphorylation of Syk. These results suggest that ER-27319 selectively inhibits the tyrosine phosphorylation of Syk induced by the interaction of Syk with the ITAM of the FcɛRI γ subunit but not with the ITAM of Igβ.
Figure 3.
Effect of ER-27319 on the phosphorylation of Syk induced by phosphorylated ITAMs or in anti-IgM stimulated human peripheral B cells. Cytosolic fractions of RBL-2H3 cell homogenates were incubated with the indicated concentration of ER-27319 (ER), 10 μM phosphorylated FcɛRI γ subunit ITAM (Pep1) (A) or Igβ ITAM (Pep2) (B) and 50 μM ATP (ATP) in kinase buffer as described in the text. (C) Human peripheral B cells were incubated in the presence (30 μM) or absence of ER-27319 before stimulation by anti-IgM. Syk was subsequently isolated with an antibody to Syk, resolved, and transferred to nitrocellulose membranes. Membranes were probed with anti-phosphotyrosine (Anti-PY) and subsequently reprobed with anti-Syk.
To further define the selectivity of ER-27319, human peripheral B cells were incubated in the presence or absence of ER-27319 and subsequently were stimulated with anti-IgM to induce tyrosine phosphorylation of Syk. Fig. 3C demonstrates that ER-27319 at 30 μM, a concentration that effectively inhibited Syk phosphorylation and degranulation of mast cells, failed to inhibit the tyrosine phosphorylation of Syk in B cells. Concentrations of up to 100 μM did not inhibit Syk phosphorylation in stimulated B cells. In addition, prolonged incubation of B cells (60 min) with ER-27319 did not inhibit Syk phosphorylation. In preliminary experiments, mast cell selectivity was further reinforced, since ER-27319 pretreatment of human platelets did not inhibit thrombin-induced platelet aggregation (data not shown).
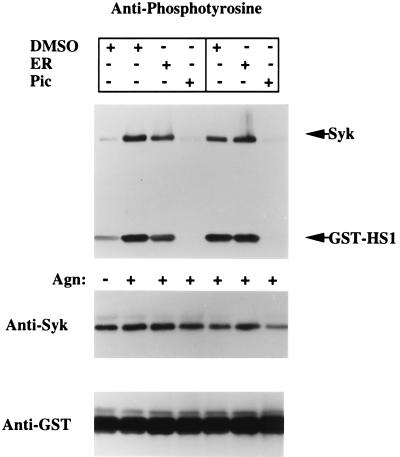
These results suggest that ER-27319 specifically inhibits the interaction of Syk with the FcɛRI γ subunit ITAM. This would predict that ER-27319 would be effective prior to activation of Syk but not subsequent to its activation. We tested this possibility by in vitro immune-complex kinase assays that utilized an in vivo substrate of Syk, GST-HS1 (24). Fig. 4 shows that pretreatment of RBL-2H3 cells with ER-27319 (30 μM) results in the inhibition of both Syk phosphorylation and activity. In three experiments, an inhibition of 66.3% ± 9.1% of the tyrosine phosphorylation of Syk was observed. Furthermore, Syk activity was inhibited by 68.7% ± 11.3%. In comparison, Syk activity from cells treated with piceatannol was completely inhibited, as was the tyrosine phosphorylation. The addition of ER-27319 (30 μM) directly to Syk immune complexes from activated cells inhibited neither the activity of Syk (Fig. 4, lane 6) nor its tyrosine phosphorylation. Concentrations of up to 100 μM had no effect on the phosphorylation of Syk and its activity (data not shown). In contrast, piceatannol completely inhibited the the tyrosine phosphorylation and activity of Syk when added to Syk immune complexes derived from activated cells (Fig. 4, lane 7). Collectively, these results show that ER-27319 selectively interferes with the activation of Syk, presumably by inhibiting its interaction with the FcɛRI γ subunit ITAM, thus inhibiting its activation. However unlike piceatannol, ER-27319 appears to have no effect on Syk once the kinase is active.
Figure 4.
ER-27319 inhibits Syk activity in activated intact cells but does not inhibit activated Syk in vitro. IgE-sensitized RBL-2H3 cells were incubated in the presence or absence of 30 μM ER-27319 (ER) or 100 nM piceatannol (Pic) before stimulation for 3 min with antigen (Agn). Cells were lysed and Syk was immunoprecipitated. Immune complexes were incubated with GST-HS1 in an in vitro kinase assay (lanes 1–4). In lanes 5–7, ER-27319 or piceatannol was added to the assay mixture containing Syk immune complexes from activated cells. Proteins were resolved by SDS/PAGE. Western blot analyses with anti-phosphotyrosine, anti-Syk, and anti-GST were performed sequentially. One of three representative experiments is shown.
DISCUSSION
The critical role of Syk in FcɛRI-mediated mast cell and basophil responses has been clearly demonstrated by the overexpression of Syk constructs with dominant-negative phenotypes that abrogated the release of allergic mediators (25). Therefore, the development of mast cell selective inhibitors of Syk may provide a reasonable approach toward therapeutic intervention in allergies. For the moment, the available tyrosine kinase inhibitors, such as genistein and herbimycin A, show little specificity for a particular protein tyrosine kinase because they function as occupants of the ATP-binding site or as competitive inhibitors of substrate interactions. Another inhibitor, piceatannol, has been demonstrated to be fairly selective for Syk in mast cells, B cells, and platelets (26–28). Because it targets Syk its use as a therapeutic agent in allergies is of interest, although it may be limited by its inhibition of B cell and platelet function. In this study we present evidence for another class of inhibitors, represented by the parental acridone-related compound ER-27319, that specifically inhibit the tyrosine phosphorylation of Syk initiated by engagement of the FcɛRI in rat and human mast cells. Our studies demonstrate that the inhibition of Syk tyrosine phosphorylation and activity by ER-27319 is mast cell selective and results in abrogation of degranulation, TNFα production, and related signaling events.
The activity of Syk is required for tyrosine phosphorylation and activation of the downstream effector PLC-γ (21). This results in the generation of inositol phosphates and diacylglycerol, leading to mobilization of intracellular Ca2+ and activation of PKC (1), resulting in secretion (29). The results of this study demonstrate that ER-27319 inhibits all of the aforementioned events we studied, consistent with the inhibition of Syk by this compound. The critical role of Syk in cell activation is not restricted to mast cells and basophils but is also important to B cell and platelet function (30, 31). In B cells, Syk activity is induced by its association with the Igα/Igβ ITAMs (23). However, our results suggest that the induction of the tyrosine phosphorylation of Syk by the Igβ ITAM is not inhibited by ER-27319, while that induced by the γ subunit ITAM is effectively inhibited. Furthermore, ER-27319 had no effect on anti-IgM-induced Syk phosphorylation in human peripheral B cells. At the moment we have no clear explanation for the mechanistic differences in the preferential inhibition of mast cell Syk phosphorylation by ER-27319, although several possibilities exist. For example, ER-27319 may bind specifically to either the FcɛRI γ subunit ITAM or to a particular region of Syk and thus inhibit the ability of Syk to bind the γ subunit ITAM and be activated by autophosphorylation or transphosphorylation. Regardless, our results clearly demonstrate that ER-27319 inhibits the activation of Syk with no effect on activated Syk. This suggests that the specificity of this drug is for the process of Syk activation. Our results in human peripheral B cells and preliminary studies on platlets show mast cell selectivity and promote the therapeutic potential of ER-27319 in allergies.
Further experiments are required to determine the site of ER-27319 binding and the stereochemical changes, if any, induced by the binding of this agent to Syk or to the γ subunit ITAM. However, preliminary experiments using biosensor technology show that ER-27319 inhibits the interaction of Syk with the FcɛRI γ subunit phospho-ITAM but only minimally affects its interaction with the Igβ phospho-ITAM (data not shown). Furthermore, additional experiments suggest that ER-27319 does not bind directly to the FcɛRI γ subunit phospho-ITAM.
Acknowledgments
We thank Dr. Michael A. Beaven (National Institutes of Health) for helpful discussions.
ABBREVIATIONS
- ER-27319
3,4-dimethyl-10-(3-aminopropyl)-9-acridone oxalate
- FcɛRI
the high-affinity receptor for IgE
- SH2
src homology domain 2, ITAM, immunoreceptor tyrosine-based activation motif
- PLC-γ1
phospholipase C-γ1
- PKC
protein kinase C
- TNFα
tumor necrosis factor α
- GST
glutathione S-transferase
- DNP
o-dinitrophenyl
- pLTs
peptide leukotrienes
References
- 1.Beaven M A, Metzger H. Immunol Today. 1993;14:222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- 2.Stevens R L, Austen K F. Immunol Today. 1989;10:381–386. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou M, Gutkind J S, Robbins K C, Siraganian R P. Proc Natl Acad Sci USA. 1990;87:5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiseman E, Bolen J B. Nature (London) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 5.Blank U, Ra C, Miller L, White K, Metzger H, Kinet J-P. Nature (London) 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 6.Reth M. Nature (London) 1989;338:383–384. [Google Scholar]
- 7.Cambier J C. J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 8.Wange R L, Malek S N, Desiderio S, Samelson L E. J Biol Chem. 1993;268:19797–19801. [PubMed] [Google Scholar]
- 9.Kihara H, Siraganian R P. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- 10.Hutchcroft J E, Geahlen R L, Deanin G G, Oliver J M. Proc Natl Acad Sci USA. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benhamou M, Ryba N J P, Kihara H, Nishikata H, Siraganian R P. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- 12.Shiue L, Green J, Green O M, Karas J L, Morgenstern J P, Ram M K, Taylor M K, Zoller M J, Zydowsky L D, Bolen J B, Brugge J S. Mol Cell Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minoguchi K, Benhamou M, Swaim W D, Kawakami Y, Kawakami T, Siraganian R P. J Biol Chem. 1994;269:16902–16908. [PubMed] [Google Scholar]
- 14.Yamada K, Jelsema C J, Beaven M A. J Immunol. 1992;149:1031–1037. [PubMed] [Google Scholar]
- 15.Holgate S T, Lewis R A, Austen K F. J Immunol. 1980;124:2093–2099. [PubMed] [Google Scholar]
- 16.Saito H, Ebisawa M, Sakaguchi N, Onda T, Iikura Y, Yanagida M, Uzumaki H, Nakahata T. Int Arch Allergy Immunol. 1995;107:63–65. doi: 10.1159/000236932. [DOI] [PubMed] [Google Scholar]
- 17.Cunha-Melo J R, Gonzaga H M S, Ali H, Huang F L, Huang K-P, Beaven M A. J Immunol. 1989;143:2617–2625. [PubMed] [Google Scholar]
- 18.Collado-Escobar D, Ali H, Beaven M A. J Immunol. 1990;144:3449–3457. [PubMed] [Google Scholar]
- 19.Germano P, Gomez J, Kazanietz M G, Blumberg P M, Rivera J. J Biol Chem. 1994;269:23102–23107. [PubMed] [Google Scholar]
- 20.Shiue L, Zoller M J, Brugge J S. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- 21.Law C L, Chandran K A, Sidorenko S P, Clark E A. Mol Cell Biol. 1996;16:1305–1315. doi: 10.1128/mcb.16.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szallasi Z, Denning M F, Chang E-Y, Rivera J, Yuspa S H, Lehel C, Olah Z, Anderson W B, Blumberg P M. Biochem Biophys Res Commun. 1995;214:888–894. doi: 10.1006/bbrc.1995.2370. [DOI] [PubMed] [Google Scholar]
- 23.Rowley R B, Burkhardt A L, Chao H G, Matsueda G R, Bolen J B. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 24.Brunati A M, Donella-Deana A, Ruzzene M, Marin O, Pinna L A. FEBS Lett. 1995;367:149–152. doi: 10.1016/0014-5793(95)00555-n. [DOI] [PubMed] [Google Scholar]
- 25.Taylor J A, Karas J L, Ram M K, Green O M, Seidel-Dugan C. Mol Cell Biol. 1995;15:4149–4157. doi: 10.1128/mcb.15.8.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver J M, Burg D L, Wilson B S, McLaughlin J L, Geahlen R L. J Biol Chem. 1994;269:29697–29703. [PubMed] [Google Scholar]
- 27.Peters J D, Furlong M T, Asai D J, Harrison M L, Geahlen R L. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- 28.Keely P J, Parise L V. J Biol Chem. 1996;271:26668–26676. [PubMed] [Google Scholar]
- 29.Ozawa K, Szallasi Z, Kazanietz M G, Blumberg P M, Mischak H, Mushinski J F, Beaven M A. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 30.Turner M, Mee P J, Costello P S, Williams O, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L. Nature (London) 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Yanagi S, Yang C, Inatome R, Yamamura H. J Biochem (Tokyo) 1997;121:325–330. doi: 10.1093/oxfordjournals.jbchem.a021590. [DOI] [PubMed] [Google Scholar]