Abstract
Many chemoattractants cause chemotaxis of leukocytes by stimulating a structurally distinct class of G protein-coupled receptors. To identify receptor functions required for chemotaxis, we studied chemotaxis in HEK293 cells transfected with receptors for nonchemokine ligands or for interleukin 8 (IL-8), a classical chemokine. In gradients of the appropriate agonist, three nonchemokine Gi-coupled receptors (the D2 dopamine receptor and opioid μ and δ receptors) mediated chemotaxis; the β2-adrenoreceptor and the M3-muscarinic receptor, which couple respectively to Gs and Gq, did not mediate chemotaxis. A mutation deleting 31 C-terminal amino acids from the IL-8 receptor type B quantitatively impaired chemotaxis and agonist-induced receptor internalization, but not inhibition of adenylyl cyclase or stimulation of mitogen-activated protein kinase. To probe the possible relation between receptor internalization and chemotaxis, we used two agonists of the μ-opioid receptor. Morphine and etorphine elicited quantitatively similar chemotaxis, but only etorphine induced receptor internalization. Overexpression of two βγ sequestering proteins (βARK-ct and αt) prevented IL-8 receptor type B-mediated chemotaxis but did not affect inhibition of adenylyl cyclase by IL-8. We conclude that: (i) Nonchemokine Gi-coupled receptors can mediate chemotaxis. (ii) Gi activation is necessary but probably not sufficient for chemotaxis. (iii) Chemotaxis does not require receptor internalization. (iv) Chemotaxis requires the release of free βγ subunits.
Keywords: chemokine receptors, receptor internalization
Inflammatory cells migrate to specific sites of infection or tissue damage in response to the localized production of chemokines. Most of these chemokines attract their targets by interacting with a structurally distinct class of G protein-coupled receptors (GPCRs) expressed on the surface of the migrating cell (1–3). Chemokine receptors initiate the temporally and spatially complex processes that detect and interpret the chemokine gradient, mobilize the appropriate cytoskeletal machinery and ultimately cause the cell to migrate in the correct direction. Remarkably little is known about the specific signaling functions required for GPCRs to mediate chemotaxis, except for the inference that these receptors activate proteins of the Gi family (4). To identify receptor functions that mediate chemotaxis, we have studied chemotaxis of cultured human embryonal kidney (HEK)293 cells expressing recombinant GPCRs and other proteins. Here we describe experiments designed to ask a series of related questions.
Pertussis toxin, which inhibits Gi signaling, blocks chemotaxis and many other responses to ligands that act on chemokine receptors (4). In addition, most chemokine receptors belong to a structurally distinct subclass of Gi-coupled receptors. Accordingly, we first ask whether nonchemokine receptors lack one or more functions uniquely required for mediating chemotaxis. Of five nonchemokine receptors tested, three mediated chemotaxis in response to appropriate ligands in 293 cells, while two did not. All three chemotaxis-competent receptors shared the ability to activate Gi proteins, whereas a Gs-coupled and a Gq-coupled receptor could not mediate chemotaxis. We conclude that the ability to mediate chemotaxis is not limited to classical chemokine receptors but instead is shared by other GPCRs that activate Gi.
Is activation of Gi sufficient, as well as necessary, for a GPCR to mediate chemotaxis? Several observations suggest that Gi activation may not suffice. For example, extracellular stimuli can trigger GPCR-mediated effects independent of G proteins (e.g., receptor phosphorylation, receptor internalization, recruitment of arrestins) (5, 6). Moreover, several well-characterized chemokine receptors [PAF, C5a, and interleukin 8 (IL-8) receptors] can activate members of the Gq family (7–9) as well as Gi. As one approach to asking whether Gi activation suffices for chemotaxis, we focused on the C terminus of a classical chemokine receptor. GPCR C termini are exposed to the cytoplasm and often play roles in mediating responses that are independent of G protein activation (10); moreover, the C terminus is an especially plausible site for a receptor function required for chemotaxis, in that migration up a chemotactic gradient may require finely tuned desensitization mechanisms or conversion of desensitization signals into migratory signals. We found that one C-terminal truncation partially inhibited chemotaxis and agonist-induced receptor internalization, but did not affect two other regulatory signals mediated by the receptor. This mutant phenotype suggests that Gi activation probably does not suffice for optimal chemotactic migration and raises the possibility that the chemotactic defect relates to impairment of receptor internalization. To test this latter notion, we compared μ-opioid receptor agonists that do or do not mediate internalization of the receptor, and found that receptor internalization is not needed for chemotaxis.
Gi-coupled receptors activate downstream effectors by generating a GTP-bound α subunit (αi⋅GTP) or by liberating free βγ. βγ triggers most of the regulatory effects mediated by these receptors; indeed, adenylyl cyclase is to date the only effector known to be regulated directly by αi⋅GTP. Moreover, release of βγ mediates the chemotrophic response to pheromone stimulation of a GPCR in Saccharomyces cerevisiae. Accordingly, we overexpressed βγ-sequestering proteins in 293 cells to ask whether βγ is an essential mediator of mammalian chemotaxis. These proteins prevented chemotaxis mediated by the IL-8 receptor type B (IL8R) but not αi-dependent inhibition of adenylyl cyclase. Thus βγ is a critical mediator of chemotactic migration.
MATERIALS AND METHODS
Materials.
Recombinant IL-8 was a gift from Caroline Hebert (Genentech). Carbachol, isoproterenol, and forskolin were purchased from Sigma. The 48-well modified Boyden Chamber was purchased from Nucleopore. [125I]-IL-8 was purchased from DuPont/NEN. Rat collagen type I was purchased from Collaborative Biomedical Products (Bedford, MA). Polycarbonate filters (10 mm) were purchased from Poretics. A mAb (M2) against the FLAG epitope was purchased from Kodak. Fluorescein isothiocyanate-conjugated antimouse IgG was obtained from Jackson ImmunoResearch.
Constructs.
cDNAs encoding the murine μ and δ opioid receptors, subcloned into pcDNA3 and FLAG- and hemagglutinin (HA)-tagged, respectively (11) and a FLAG-tagged human β2-adrenoreceptor, were provided by Mark von Zastrow (University of California, San Francisco). A cDNA for the rat M3-muscarinic receptor, containing an HA-tag at the N terminus (12), was provided by Jurgen Wess (National Institute of Diabetes and Digestive and Kidney Diseases). HA-tagged p44 extracellular signal-regulated kinase (p44ERK; ERK1) was a gift from J. Pouysségur (Centre National de la Recherche Scientifique, Nice, France) as described (13). The cDNA for the D2 dopamine receptor was obtained as noted (14). The cDNA encoding βARK-ct-(495–689) was obtained from Robert Lefkowitz (Duke University, Durham, NC).
Cell Culture and Transfection.
HEK293 cell lines (15) were grown in DMEM with Earle’s balanced salt solution supplemented with 10% fetal calf serum. For stable transfections, cells were grown to 50–70% confluence and transfected, by calcium phosphate precipitation, with 10–20 μg of DNA containing a neomycin-selectable marker. Stable clones were selected (16) and maintained in G418 (800 μg/ml). For transient transfection with the ERK construct, the DEAE/dextran method was used as described (14). Forty-eight hours after transfection, cells were serum starved for 16 hr in preparation for the ERK activation assay.
Chemotaxis Assay.
Cell migration assays were performed as described (15). The polycarbonate filters were coated with 50 μg/ml of rat collagen type I for 2 hr at 37°C. The chambers were incubated for 3–4 hr at 37°C in humidified air with 5% CO2. For each agonist concentration tested, cells that had migrated through to the underside of the filter were counted in four high-power fields (400×), in a blinded fashion. The migration index for each experiment was the mean number of cells that migrated at a specific agonist concentration divided by mean number of cells that migrated toward medium containing BSA. For each migration assay, a chemokinesis control using the optimal chemotactic concentration on both sides of the filter was performed to insure that the observed migration reflected chemotaxis and not chemokinesis. In no case did chemokinesis account for >15% of total cell migration (data not shown).
cAMP Accumulation.
Cells were split into 24-well plates at a density of 3–5 × 105 cells per well and labeled for 24 hr with medium containing 2 μCi/ml [3H]-adenine (23 Ci/mmol; 1 Ci = 37 GBq, Amersham). The next day, the cells were stimulated with forskolin in the presence or absence of the appropriate agonists. cAMP accumulation was determined as described (17).
ERK Activity.
HA-ERK1 DNA was transfected into the stable cell lines as described above. HA-ERK activity was measured as described (13). Radioactivity incorporated into myelin basic protein was determined by PhosphorImager analysis.
Inositol Phosphate Accumulation.
Subconfluent cells were split into a 24-well plate and labeled with [3H]-inositol (2 μCi/ml) for 24 hr. The next day, the cells were washed once with assay medium and then incubated with assay medium containing 10 mM LiCl with or without the appropriate agonist. After a 30-min incubation at 37°C, the cells were lysed in 20 mM formic acid and assayed as described (14).
Receptor Binding and Internalization.
Cells were plated in 12-well dishes 1–2 days before the binding assay. When the cells were confluent, [125I]-IL-8 was added at a concentration of 1 nM (30,000–50,000 cpm per well). The cells were incubated at 4°C for 2 hr, washed twice with buffer, and then solubilized in lysis buffer containing 0.25% SDS and 0.25 M NaOH. Radioactivity was quantified in a γ counter. Nonspecific binding was determined in the presence of 100 nM nonradioactive IL-8.
For measuring ligand internalization, we used an acid washing procedure. After incubating the cells with tracer isotope (1 nM) at 4°C for 2 hr, the cells were washed with buffer, and transferred to an incubator at 37°C for the specified time intervals. The cells were then placed on ice and washed again with an acid wash solution containing 5 mM acetic acid in 0.9% saline, pH 2.5. This removed >90% of surface counts (data not shown). The cells were solubilized and internalized counts were measured. Percent internalization was quantified as the percent of total binding counts that remained after the acid wash procedure.
RESULTS
Nonchemokine Receptors.
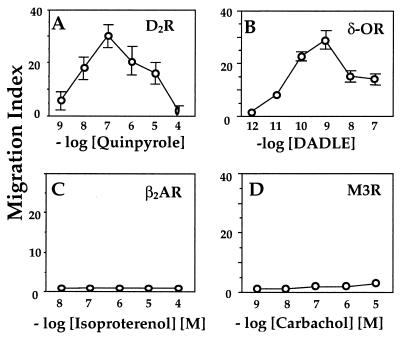
To ask whether classical chemokine receptors are unique in their ability to transmit chemotactic signals, we studied five GPCRs that are not classical chemokine receptors and whose primary structures do not resemble those of canonical chemokine receptors. Three Gi-coupled receptors (the D2 dopamine receptor and the μ and δ opioid receptors) supported chemotaxis (Figs. 1 A and B; see Fig. 5A). Compared with IL-8-induced chemotaxis of IL8R-expressing cells (Fig. 2), however, migration indices of the cells expressing nonchemokine receptors were generally lower (<50%; for example, compare Fig. 1A vs. Fig. 2A). Nonetheless, the nonchemokine receptors mediated agonist inhibition of adenylyl cyclase as well as did the IL8R, within dose ranges that were optimal for chemotaxis (data not shown).
Figure 1.
Chemotaxis of cells expressing nonchemokine receptors. Migration assays were performed in a 48-well Boyden chamber, as described (15) on stable transfectants expressing (A) D2 dopamine receptor, (B) HA-tagged δ opioid receptor, (C) FLAG-tagged β2-adrenoreceptor, and (D) HA-tagged M3-muscarinic receptor. Values represent the mean ± SE of six determinations. Similar results were obtained in three or more independent experiments. In these and all other migration assays, chemokinesis controls (described in Materials and Methods) showed that chemokinesis could not account for >15% of total cell migration (data not shown).
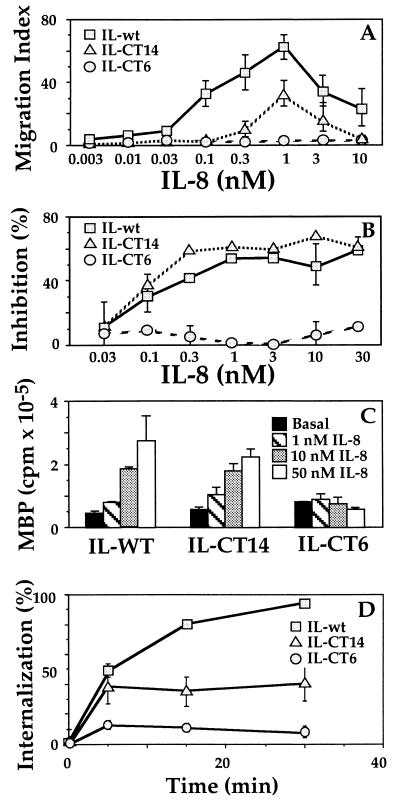
Figure 2.
Wild-type and C-terminal truncation mutants of the IL-8 receptor. (A) Migration assays, performed as described in the legend to Fig. 1, on cells expressing C-terminal truncation mutants (IL-CT14 or IL-CT6) or the wild-type IL-8 receptor (15). (B) cAMP accumulation. Cells were incubated with 200 μM forskolin and the indicated concentrations of IL-8. Values represent percent inhibition by IL-8 of the forskolin-stimulated cAMP response, measured as described (17). Forskolin increased cAMP >100-fold over basal. (C) MAPK (ERK) activation. Stable transfectants were transiently transfected with 1 μg HA-ERK1 DNA and after 48 hr ERK activities were assessed as described (13). ERK activation is expressed in PhosphorImager units. (D) Receptor internalization. The percent of surface receptors remaining after various times of exposure to agonist was measured in whole-cell binding assays using [125I]-IL-8. Data shown represents the mean ± SE of triplicate determinations. Results similar to those in A–D were obtained in three or more independent experiments. HEK293 cell lines expressing the wild-type and mutant IL8Rs were obtained from Adit Ben-Baruch (Tel Aviv University) (15).
Neither the β2-adrenoreceptor nor the M3 muscarinic receptor, which couple to Gs and Gq, respectively, promoted chemotaxis (Figs. 1 C and D); in control experiments, these receptors mediated robust agonist-stimulated accumulation of cAMP and inositol phosphate, respectively (data not shown). These data show that Gi-coupled receptors (chemokine and nonchemokine) can mediate chemotaxis and that neither Gq nor Gs can substitute for Gi in inducing the chemotactic response.
IL-8 Receptor Mutants.
We tested three previously reported cell lines, which stably express the wild-type IL8R (type B) and two C-terminal truncation mutants. Of 45 C-terminal residues, the IL-CT14 mutant retained 14 and the IL-CT6 mutant 6. None of the potential C-terminal phosphorylation sites (eight serines and threonines) remains in either truncated receptor. As reported (15), these three cell lines expressed similar numbers of receptors (within a 3-fold range) and the receptors showed similar affinities for binding IL-8. Chemotaxis assays (Fig. 2A) confirmed previous observations (15). Specifically, compared with the wild-type receptor, the IL-CT14 mutant demonstrated a 50% decrease in maximal migration index and a seven-fold increase in the EC50 for migration. The data shown represents the mean ± SE of triplicate determinations. CT6 mutant did not migrate in response to IL-8. Pertussis toxin blocked chemotaxis mediated by the wild-type receptor, suggesting that Gi signaling was required (data not shown).
Do the chemotaxis defects in the mutants reflect defects in G protein signaling? The IL-CT14 mutant activated mitogen-activated protein kinase (MAPK) and inhibited forskolin-stimulated adenylyl cyclase activity with maximal effects and EC50/IC50 values similar to the wild-type receptor (Figs. 2 B and C). The IL-CT6 mutant did not signal (Figs. 2 B and C). These data suggested that the C terminus of the IL8R is critical for both G protein activation and chemotaxis, and that maximally efficient chemotaxis requires some function that is impaired by the IL-CT14 mutation but that is not required for maximally effective inhibition of adenylyl cyclase or activation of MAPK.
Internalization and Chemotaxis.
Because the C terminus is involved in receptor internalization and phosphorylation, we asked whether the observed chemotaxis defects in the IL-8 receptor mutants might be linked to defects in internalization. For each mutant, receptor internalization and chemotaxis were impaired to a similar degree—that is, both responses were partially defective for IL-CT14 and completely absent for IL-CT6 (compare Fig. 2 A vs. D). Our findings are consistent with the reported phenotype (18) of a C-terminal mutant of the IL-8 receptor type A (comparable to the IL-CT14 mutant in our experiments), which showed a significant defect in agonist-mediated receptor internalization. Thus, it appeared that chemotaxis could require internalization or share one or more downstream signaling components with the internalization process.
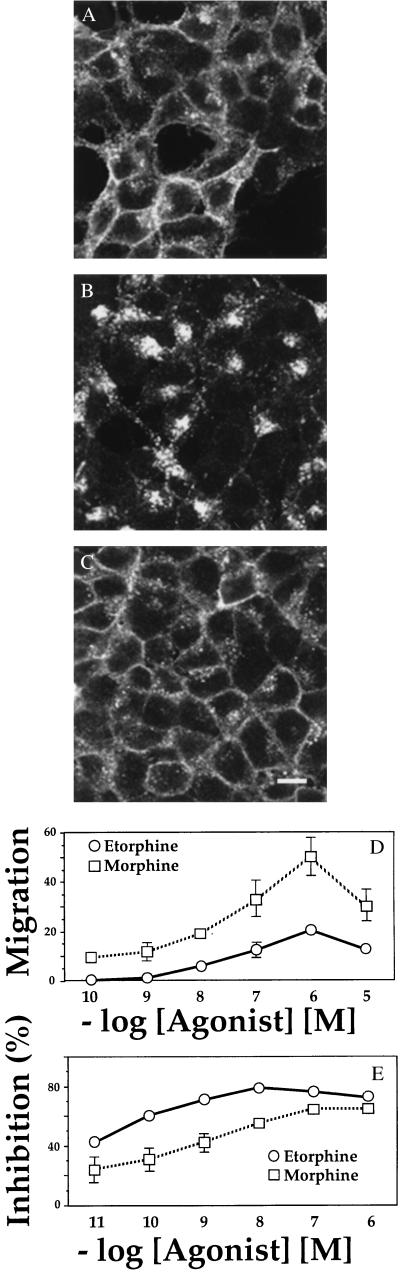
To test more rigorously whether internalization is coupled to chemotaxis, we studied cells transfected with the μ-opioid receptor. Recent reports (11, 19) indicate that some peptide and alkaloid opioid agonists induce efficient internalization of opioid receptors, while other alkaloid agonists, such as morphine, do not. Using immunofluorescence and confocal imaging to gauge agonist-mediated receptor internalization, we confirmed that etorphine promoted marked internalization of the μ-opioid receptor at 1 hr and that morphine did not promote detectable internalization (Figs. 3 A–C). Both agonists induced robust agonist-mediated chemotaxis, however, with a migration index that was greater for morphine than for etorphine (Fig. 3D). Both agonists also inhibited adenylyl cyclase, confirming that they activated Gi (Fig. 3E).
Figure 3.
Responses of cells expressing the μ opioid receptor. (A–C). Immunofluorescence localization. Cells stably expressing a FLAG-tagged μ-opioid receptor were incubated for 1 hr in the absence of ligand (A) or in the presence of 100 nM etorphine (B) or 100 nM morphine (C), then fixed in 4% formaldehyde. The receptor was detected by incubation with the monoclonal antibody M2, followed by fluorescein isothiocyanate-conjugated anti-mouse antibody (16). Specimens were examined by confocal microscopy. Scale bar = 10 μm. (D) Chemotaxis, assessed as in Fig. 1, in response to morphine or etorphine. Similar results were obtained in three separate experiments. Values represent the mean ± SE of six determinations. (E) Inhibition of forskolin-stimulated cAMP accumulation was measured in response to increasing concentrations of morphine or etorphine, as in Fig. 2B. Similar results were obtained in three separate experiments. Bars = mean ± SE of three determinations.
βγ and Chemotaxis.
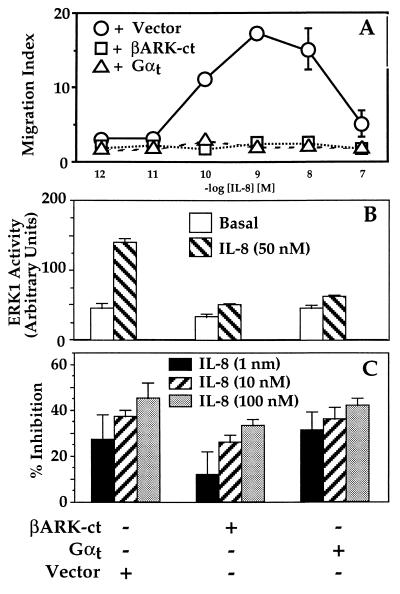
Ligands for Gi coupled receptors can activate downstream effectors either by generating αi⋅GTP or by liberating free βγ. To test whether βγ plays an essential role in chemotaxis, we measured chemotaxis in HEK293 cells stably coexpressing the IL-8 receptor and either of two proteins that sequester βγ and block Gi signals mediated by βγ. These are a C-terminal fragment of the β-adrenoreceptor kinase, βARK-ct-(495–689) (20, 21) and the α subunit (αt) of the retinal G protein, transducin (22). Each protein completely prevented chemotaxis in response to IL-8 (Fig. 4A). The same proteins inhibited IL-8-induced activation of MAPK (Fig. 4B), a βγ-dependent response (13, 20–24), but did not inhibit IL-8-induced inhibition of cAMP accumulation (Fig. 4C), a response that is mediated by the αi subunit (25).
Figure 4.
Responses to IL-8 in cells expressing proteins that sequester βγ. (A) Chemotaxis. Migration assays were performed as described in the legend to Fig. 1 on cells expressing the wild-type IL-8 receptor stably cotransfected with either pcDNA1, pRK-βARK-1-(495–689) (βARK-ct), or αt-pcDNA1, each in combination with a plasmid encoding a hygromycin-resistance marker. (B) MAPK (ERK) activation. Double transfectants were analyzed as described in the legend to Fig. 2. ERK1 activity is expressed in PhosphorImager units. Epidermal growth factor caused 4- to 5-fold activation of ERK in each cell line. (C) Inhibition of cAMP accumulation. Transfectants were incubated with 200 μM forskolin and the indicated concentrations of IL-8. Bars = percent inhibition by IL-8 of the forskolin-stimulated cAMP response. In cells treated with forskolin alone, cAMP accumulation over basal values (measured as the ratio of [3H]cAMP to the sum of [3H]cAMP plus [3H]ATP) was 115, 160, or 247 × 10−3 in cells expressing pcDNA1, βARK-ct, or αt. Results similar to those in each panel were obtained in at least two independent experiments. Data shown represent the mean ± SE of triplicate determinations for A and C and the mean and range of duplicate determinations for B.
DISCUSSION
Investigation of chemotaxis has identified a growing number of chemoattractants for the cells responsible for inflammatory and immune responses (1, 2). Most of these chemoattractants act on a structurally distinct class of chemokine receptors that are coupled to G proteins. Here we report initial experiments aimed at identifying functions of GPCRs that are required to mediate chemotaxis. Our results support four inferences: (i) Receptors coupled to Gi can mediate chemotaxis even when they lack the distinguishing features of chemokine receptors and when their agonist ligands are not classical chemokines. (ii) Activation of Gi is required but probably not sufficient for chemotaxis. (iii) Receptors can mediate chemotaxis without undergoing agonist-induced internalization. (iv) βγ is an essential mediator of chemotaxis.
Do chemokine receptors differ in some characteristic way from other GPCRs? The complexity of chemotaxis suggests that it may be mediated by receptors with specialized functions. Although chemotactic responses in many leukocytes require activation of Gi, as indicated by their well-known sensitivity to inhibition by pertussis toxin (4), leukocytes express Gi-coupled receptors (e.g., serotonin, somatostatin) that are not known to induce chemotaxis (26, 27). Thus it is reasonable to ask whether chemotaxis requires receptors that are able to generate additional signals independent of Gi—in other words, whether Gi activation is not sufficient, although it is necessary, for chemotaxis. In addition, sensitivity of leukocyte chemotaxis to pertussis toxin could reflect the limited repertoire of receptors and G proteins expressed in leukocytes; if so, receptors coupled to G proteins other than Gi might mediate chemotaxis in the appropriate cell type. Such questions were the focus of our first experiments (Fig. 1). The results showed that nonchemokine Gi-coupled receptors could mediate chemotaxis, while nonchemokine Gs- and Gq-coupled receptors could not (Fig. 1 A and B vs. C and D). Thus, receptors’s ability to activate Gi is critical for inducing directional migration toward an agonist, but distinctive structural features of a subclass of chemoattractant receptors are not.
These observations raise a critical question: What are the key signals transmitted by Gi-coupled receptors but not by other GPCRs? We approached this question in two ways. First, using C-terminal mutants of the IL8R, we asked whether Gi signaling is both necessary and sufficient for chemotaxis and whether C-terminal mutations affect specific receptor signals (dependent on or independent of Gi) that might mediate the chemotactic response. Second, we assessed chemotaxis in 293 cells over-expressing βγ-sequestering proteins, to ask whether βγ released from Gi is responsible for chemotaxis mediated by the IL-8 receptor.
The next set of observations showed that Gi activation is necessary probably does not suffice for a GPCR to mediate chemotaxis. From the phenotype of the IL-CT14 mutant we infer that optimally efficient chemotaxis depends on functional properties of chemokine receptors that are distinct from the ability to activate Gi. This mutant receptor can trigger both inhibition of adenylyl cyclase (by αi⋅GTP) and ERK activation (βγ-mediated) just as well as the wild-type IL8R, but shows a reproducible defect in mediating chemotaxis (Fig. 2); the chemotactic response mediated by this receptor would have been normal if Gi-dependent signals were sufficient. Supporting the same inference, migration indices seen with agonists for nonchemokine Gi-coupled receptors were generally lower than those observed with the IL8R, a “professionally” chemotactic receptor (compare Fig. 2A vs. Figs. 1 A and B, and 3D).
The ILCT14 receptor mediated an αi⋅GTP-dependent and a βγ-dependent response indistinguishable from those produced by the wild-type IL8R (Fig. 2), but showed parallel defects in both chemotaxis and agonist-induced receptor internalization. Taken together, these observations raise the possibility that signaling pathways responsible for chemotaxis share common elements with the G protein-independent signal(s) responsible for receptor internalization. A report (28) that pharmacological inhibition of IL8R internalization also inhibits chemotaxis is in accord with this idea. We found, however, that the μ-opioid receptor mediates chemotaxis toward a μ-opioid agonist that does not stimulate receptor internalization, indicating that chemotaxis does not require receptor internalization. In accord with this negative inference, Arai et al. recently showed that mutational replacement of C-terminal phosphorylation sites in the MCP-1 receptor inhibits receptor internalization by >40% but preserves chemotactic migration (29). Thus receptor internalization itself is not necessary for chemotaxis, although chemotaxis and the internalization response may share essential signaling elements (for instance, proteins like the arrestins or overlapping receptor sequences important for both processes).
Which subunit of the Gi heterotrimer is required for chemotactic migration? Because chemotrophic responses in S. cerevisiae require liberation of βγ, we specifically asked whether βγ plays an essential role in chemotaxis in 293 cells. Sequestration of βγ by αt and by a βARK fragment prevented chemotaxis (Fig. 4). These results clearly indicate that βγ is also an essential downstream mediator in mammalian chemotaxis (although they does not rule out an additional role for αi⋅GTP; see below). What effector proteins are directly regulated by βγ to mediate the chemotactic response? The answer is unknown, although several known βγ effectors (summarized in ref. 30) are unlikely to play key roles. The unlikely candidates include an isoform of phospholipase Cβ, PI3 kinase, and p42/p44 MAPKs (13, 23, 30, 31). For example, the β2 isoform of phospholipase C is thought to mediate Gi-dependent activation of phosphoinositide synthesis and elevation of intracellular Ca2+ in neutrophils (reviewed in ref. 32), but a transgenic knockout of PLCβ2 produces mice whose neutrophils migrate more effectively toward chemoattractants than do neutrophils of appropriate controls (32). Similarly, an inhibitor of ERK kinase did not affect chemotaxis of neutrophils (33); the inhibitor also failed to inhibit chemotaxis of HEK293 cells (result not shown). Different laboratories have reported that wortmannin, an inhibitor of PI3 kinase, inhibits (33) or does not (34) inhibit neutrophil chemotaxis; wortmannin did not affect chemotaxis of 293 cells (data not shown). These negative results suggest that as yet undiscovered βγ effectors may be involved in the chemotactic response.
What does the α subunit of Gi contribute to the chemotactic response? Because inhibition of adenylyl cyclase is the only known direct effect of αi⋅GTP, one might surmise that changes in the intracellular concentration of cAMP are important for chemotaxis. Chemotactic stimulation of neutrophils results in transient cAMP elevation and chemokines stimulate adenylyl cyclase in T lymphocytes (35, 36). We found, however, that the β2AR (which activates Gsα, a direct activator of adenylyl cyclase) did not stimulate chemotaxis (Fig. 1C) and that forskolin treatment (a chemical activator of adenylyl cyclase) inhibited IL-8-stimulated chemotaxis of cells expressing the wild-type IL8R (data not shown). The most coherent interpretation of these observations is that cAMP is not a critical intracellular mediator of chemotaxis and that extreme or persistent cAMP elevations can be inhibitory. Other undiscovered effectors of Giα might, in combination with βγ, support chemotaxis, which probably depends on multiple signaling pathways that converge to produce directional migration.
Inferences drawn from our experiments are limited by the receptors we tested and by the experimental model we used. For example, consider the possibility that our selection of nonchemokine receptors was biased toward inclusion of a subset of Gi-coupled receptors that mediate chemotactic signals. This is unlikely in the case of the D2 dopamine receptor, which has not (to our knowledge) been identified on neutrophils or other chemotactic cells. Similarly, the μ-opioid receptor tested in our experiments has not been described on leukocytes. Although recent investigations (37, 38) have described responses of hematopoietic cells mediated by receptors that pharmacologically resemble the δ-opioid receptor, the δ-opioid agonists caused both inhibitory and stimulatory effects on chemotaxis (37, 38), indicating that this receptor is not a classical chemokine receptor.
It is unlikely that chemotaxis-mediating pathways in HEK293 cells, an epithelial cell line, are identical in detail to those of professionally chemotactic cells, such as blood neutrophils. Indeed, the cell types differ markedly in morphology, in adherence to glass, and speed of migration in the Boyden chamber assay (minutes for neutrophils vs. several hours for 293 cells). Further experiments will be required to determine the degree to which chemotaxis in 293 cells resembles that of neutrophils, beyond the shared sensitivity to inhibition by pertussis toxin.
Finally, our experiments raise fascinating questions: What are the putative mediators of chemotaxis that act independently of G protein activation? What downstream signaling elements link βγ to chemotaxis? How do these complex pathways interact to produce the spatially and temporally complex remodeling of the actin cytoskeleton required for cell polarization and motility. Experiments in this and other model systems will soon begin to tackle these questions.
Acknowledgments
We thank Israel Charo, Shaun Coughlin, Joost Oppenheim, and members of the Bourne laboratory for useful advice and review of the manuscript. E.N. is a fellow supported in part by fellowships from the Howard Hughes Medical Institute, the Robert Wood Johnson Foundation, the University of California, San Francisco, Molecular Medicine Training Program, and National Institutes of Health Training Grant T32HL-07185. This work is supported in part by National Institutes of Health Grants GM-27800 and CA-54427 (to H.R.B.).
ABBREVIATIONS
- HA
hemagglutinin
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- GPCRs
G protein-coupled receptors
- IL-8
interleukin 8
- IL8R
IL-8 receptor type B
- HEK
human embryonal kidney
References
- 1.Baggiolini M, Dewald B, Moser B. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Murphy P M. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 3.Premack B A, Schall T J. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 4.Gerard C, Gerard N P. Curr Opin Immunol. 1994;6:140–145. doi: 10.1016/0952-7915(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 5.Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–351. ; discussion, 352–353. [PubMed] [Google Scholar]
- 6.Ferguson S S, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- 7.Wu D, LaRosa G J, Simon M I. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 8.Amatruda T T d, Gerard N P, Gerard C, Simon M I. J Biol Chem. 1993;268:10139–10144. [PubMed] [Google Scholar]
- 9.Buhl A M, Eisfelder B J, Worthen G S, Johnson G L, Russell M. FEBS Lett. 1993;323:132–134. doi: 10.1016/0014-5793(93)81464-b. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 11.Keith D E, Murray S R, Zaki P A, Chu P C, Lissin D V, Kang L, Evans C J, von Zastrow M. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 12.Schoneberg T, Liu J, Wess J. J Biol Chem. 1995;270:18000–18006. doi: 10.1074/jbc.270.30.18000. [DOI] [PubMed] [Google Scholar]
- 13.Faure M, Voyno-Yasenetskaya T A, Bourne H R. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 14.Conklin B R, Farfel Z, Lustig K D, Julius D, Bourne H R. Nature (London) 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Baruch A, Bengali K M, Biragyn A, Johnston J J, Wang J-M, Kim J, Chuntharapai A, Michiel D F, Oppenheim J J, Kelvin D J. J Biol Chem. 1995;270:9121–9128. doi: 10.1074/jbc.270.16.9121. [DOI] [PubMed] [Google Scholar]
- 16.Wedegaertner P B, Bourne H R, von Zastrow M. Mol Biol Cell. 1996;7:1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong Y H, Federman A, Pace A M, Zachary I, Evans T, Pouysségur J, Bourne H R. Nature (London) 1991;351:63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- 18.Prado G N, Suzuki H, Wilkinson N, Cousins B, Navarro J. J Biol Chem. 1996;271:19186–19190. doi: 10.1074/jbc.271.32.19186. [DOI] [PubMed] [Google Scholar]
- 19.Sternini C, Spann M, Anton B, Keith D E, Jr, Bunnett N W, von Zastrow M, Evans C, Brecha N C. Proc Natl Acad Sci USA. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch W J, Hawes B E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch W J, Hawes B E, Inglese J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 22.Lustig K D, Conklin B R, Herzmark P, Taussig R, Bourne H R. J Biol Chem. 1993;268:13900–13905. [PubMed] [Google Scholar]
- 23.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 24.Pace A M, Faure M, Bourne H R. Mol Biol Cell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taussig R, Iñiguez-Lluhi J A, Gilman A G. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 26.Bondesson L, Nordlind K, Liden S, Sundstrom E. Immunopharmacol Immunotoxicol. 1993;15:243–250. doi: 10.3109/08923979309025997. [DOI] [PubMed] [Google Scholar]
- 27.Carolan E J, Casale T B. J Allergy Clin Immunol. 1993;92:589–598. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 28.Ray E, Samanta A K. FEBS Lett. 1996;378:235–9. doi: 10.1016/0014-5793(95)01462-4. [DOI] [PubMed] [Google Scholar]
- 29.Arai H, Monteclaro F S, Tsou C, Franci C, Charo I. J Biol Chem. 1997;272:25037–25042. doi: 10.1074/jbc.272.40.25037. [DOI] [PubMed] [Google Scholar]
- 30.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 31.Stephens L, Smrcka A, Cooke F T, Jackson T R, Sternweis P C, Hawkins P T. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Kuang Y, Wu Y, Wei X, Simon M I, Wu D. Proc Natl Acad Sci USA. 1997;94:7971–7975. doi: 10.1073/pnas.94.15.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knall C, Worthen G S, Johnson G L. Proc Natl Acad Sci USA. 1997;94:3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thelen M, Uguccioni M, Bosiger J. Biochem Biophys Res Commun. 1995;217:1255–1262. doi: 10.1006/bbrc.1995.2903. [DOI] [PubMed] [Google Scholar]
- 35.del Pozo M A, Sanchez-Mateos P, Nieto M, Sanchez-Madrid F. J Cell Biol. 1995;131:495–508. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elferink J G R, VanUffelen B E. Gen Pharmacol. 1996;27:387–393. doi: 10.1016/0306-3623(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 37.Makman M H. Adv Neuroimmunol. 1994;4:69–82. doi: 10.1016/s0960-5428(05)80002-6. [DOI] [PubMed] [Google Scholar]
- 38.Sibinga N E, Goldstein A. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]