Abstract
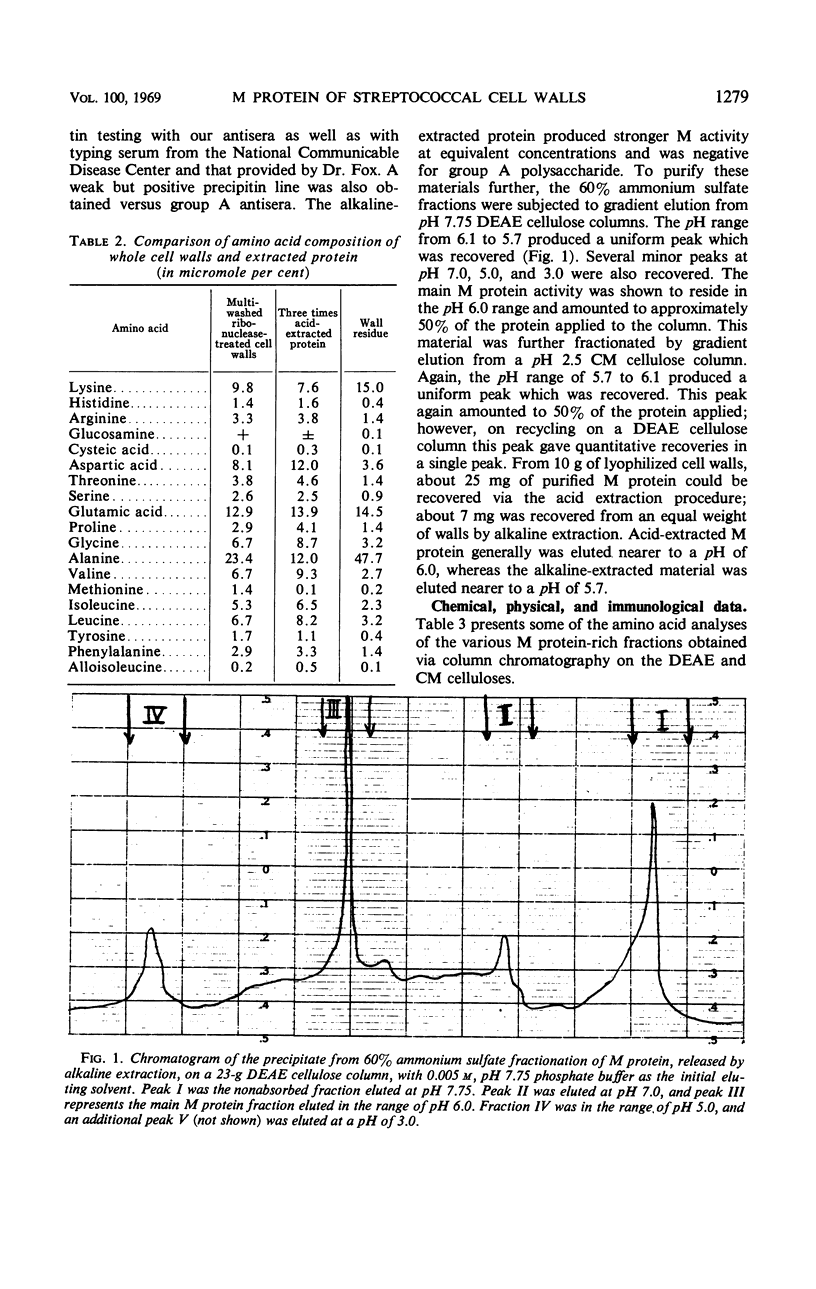
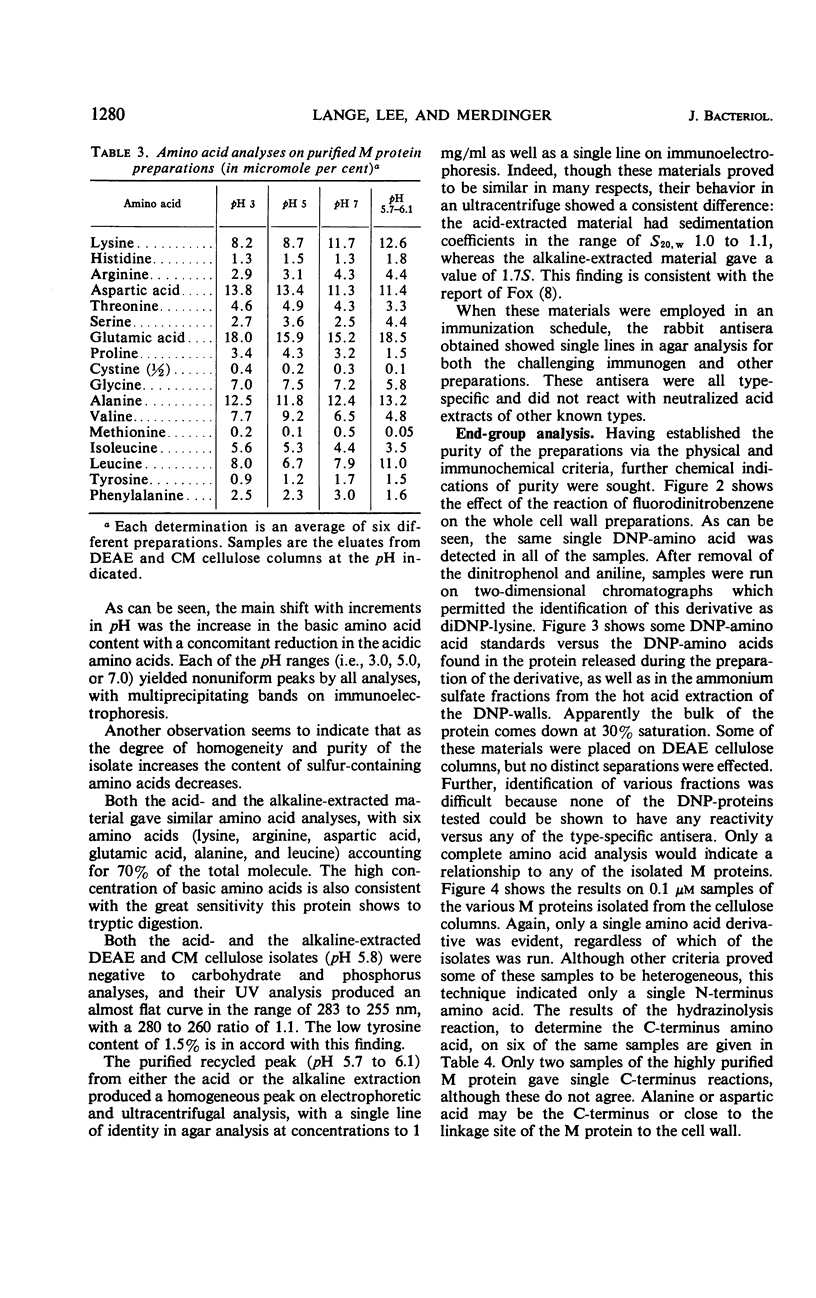
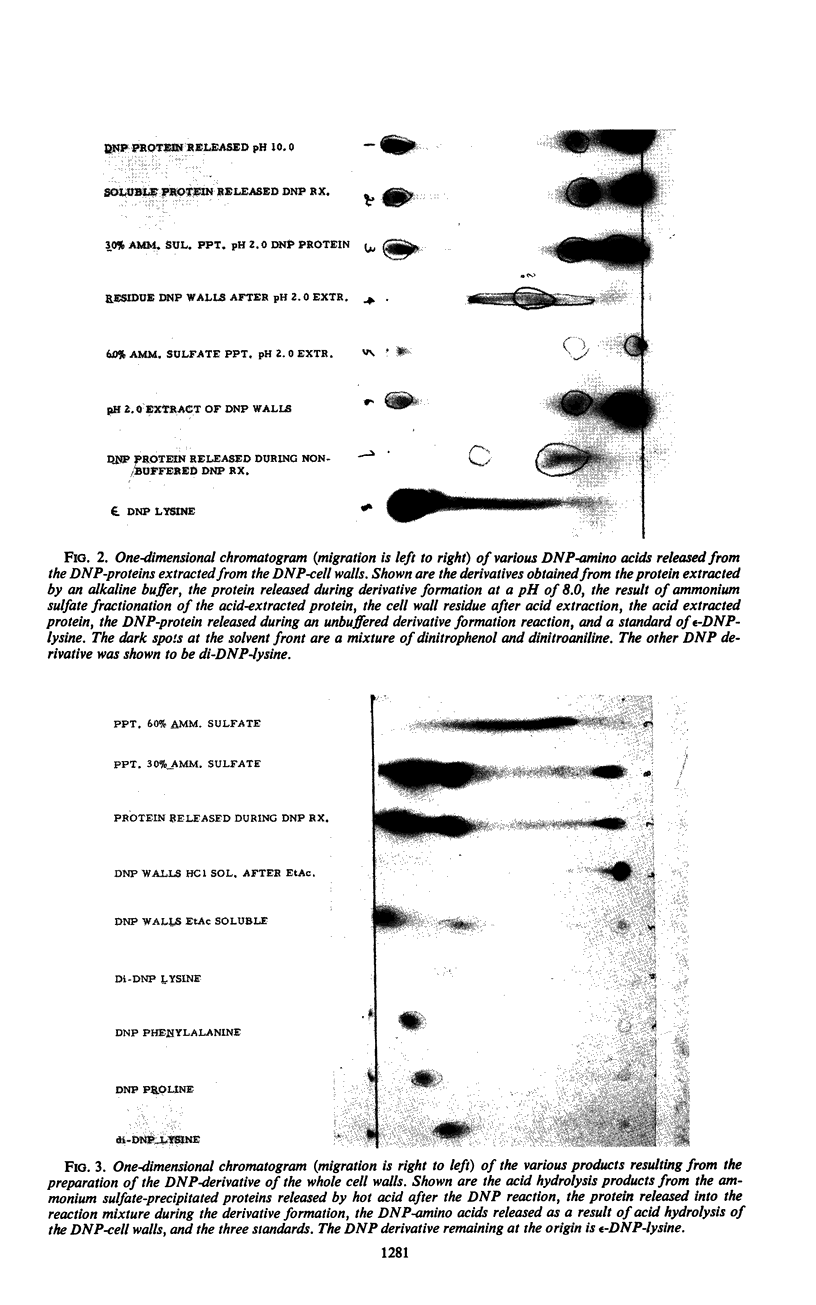
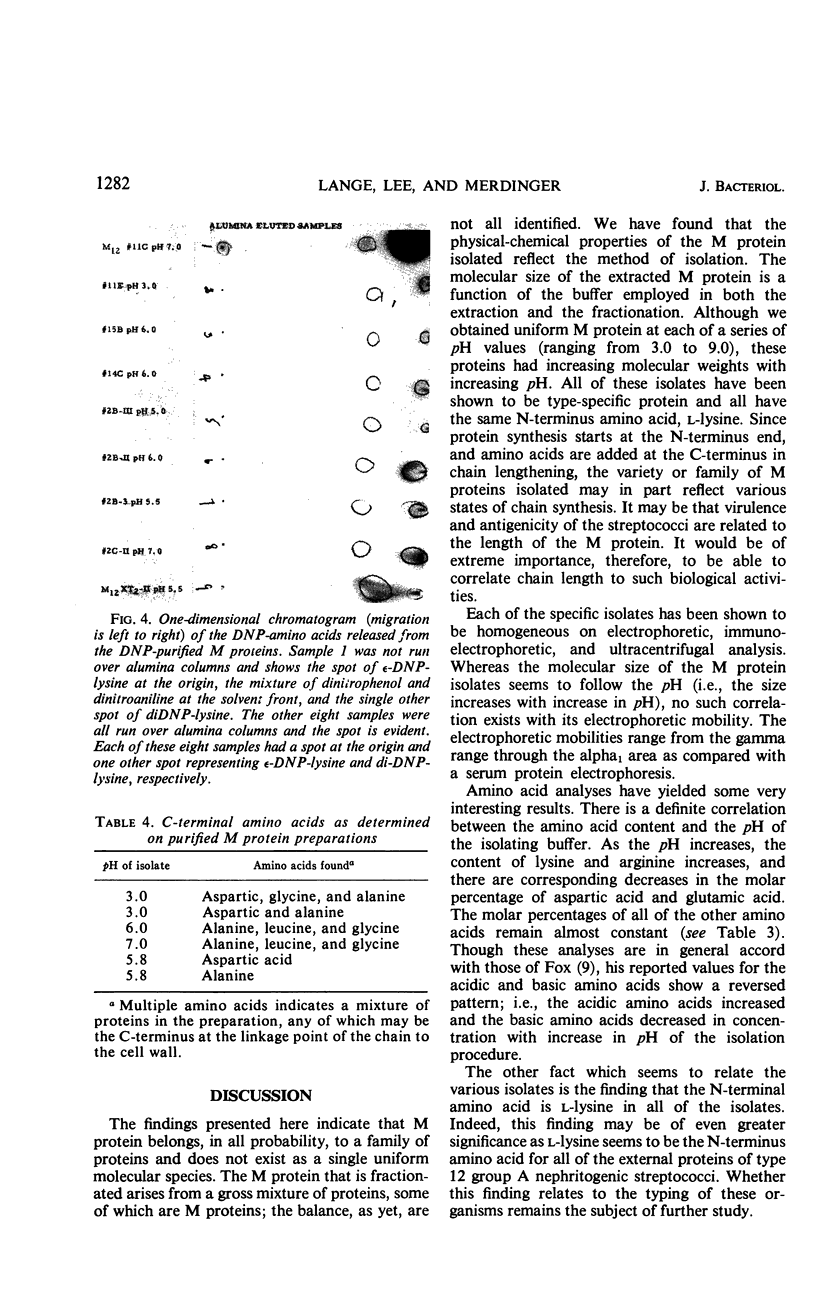
M protein was extracted from the cell walls of streptococci by use of both acidic and alkaline buffers. These extracts were further purified by ammonium sulfate fractionation and column chromatography. Both diethylaminoethyl and carboxymethyl celluloses were employed to cover the pH range of 3.0 to 9.0. All of the M proteins isolated were immunologically related, but their physical and chemical properties varied dependent upon the pH range of isolation. Each isolate appeared to be homogeneous on the basis of immunodiffusion analysis, electrophoretic mobility, and ultracentrifugal analysis, but their amino acid analyses differed slightly. Two factors were shared by all isolates: (i) they all reacted with type-specific antisera and (ii) each seemed to have l-lysine as a single N-terminal amino acid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S., JONES M. F. Studies of streptococcal cell walls. I. Isolation, chemical composition, and preparation of M protein. J Bacteriol. 1957 Aug;74(2):207–216. doi: 10.1128/jb.74.2.207-216.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N. ANTIGENICITY OF THE M PROTEINS OF GROUP A HEMOLYTIC STREPTOCOCCI. J Immunol. 1964 Nov;93:826–837. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., PORTER R. R. The terminal amino groups of conalbumin, ovomucoid and avidin. Biochim Biophys Acta. 1952 Nov;9(5):557–562. [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. Antigenicity of the M proteins of group A hemolytic streptococci. II. Antibody response in rabbits to vaccines prepared with oil emulsions and aluminum hydroxide. J Immunol. 1966 Jul;97(1):86–94. [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K., Dorfman A. Antigenicity of the M proteins of group A hemolytic streptococci. 3. Antibody responses and cutaneous hypersensitivity in humans. J Exp Med. 1966 Dec 1;124(6):1135–1151. doi: 10.1084/jem.124.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. New observations on the structure and antigenicity of the M proteins of the group A streptococcus. Immunochemistry. 1969 Jan;6(1):11–24. doi: 10.1016/0019-2791(69)90174-8. [DOI] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. The multiple molecular structure of the M proteins of group A streptococci. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1118–1125. doi: 10.1073/pnas.54.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN J. J., COLE R. M. STREPTOCOCCAL M ANTIGEN LOCATION AND SYNTHESIS, STUDIED BY IMMUNOFLUORESCENCE. J Exp Med. 1963 Nov 1;118:659–666. doi: 10.1084/jem.118.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lancefield R. C. Current problems in studies of streptococci. J Gen Microbiol. 1969 Feb;55(2):161–163. doi: 10.1099/00221287-55-2-161. [DOI] [PubMed] [Google Scholar]
- Lange C. F. A method for enhancing visualization of precipitin lines in agar analysis. Clin Chim Acta. 1967 Oct;18(1):91–92. doi: 10.1016/0009-8981(67)90255-0. [DOI] [PubMed] [Google Scholar]
- MARKOWITZ A. S., LANGE C. F., Jr STREPTOCOCCAL RELATED GLOMERULONEPHRITIS. I. ISOLATION, IMMUNOCHEMISTRY AND COMPARATIVE CHEMISTRY OF SOLUBLE FRACTIONS FROM TYPE 12 NEPHRITOGENIC STREPTOCOCCI AND HUMAN GLOMERULI. J Immunol. 1964 Apr;92:565–575. [PubMed] [Google Scholar]
- Markowitz A. S. RAPID PRODUCTION OF ANTI-M PROTEIN ANTIBODIES. J Bacteriol. 1963 Feb;85(2):495–496. doi: 10.1128/jb.85.2.495-496.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE W. A., Jr ELECTROPHORETIC SEPARATION OF CONSTITUENTS OF PARTIALLY PURIFIED M PROTEIN OF STREPTOCOCCUS PYOGENES. J Bacteriol. 1964 Oct;88:912–921. doi: 10.1128/jb.88.4.912-921.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- TURBA F., GUNDLACH G. Abtrennung des Dinitrophenols von Dinitrophenyl-Aminosäuren und -Peptiden. Biochem Z. 1955;326(5):322–324. [PubMed] [Google Scholar]