Abstract
Chemotaxis is mediated by activation of seven-transmembrane domain, G protein-coupled receptors, but the signal transduction pathways leading to chemotaxis are poorly understood. To identify G proteins that signal the directed migration of cells, we stably transfected a lymphocyte cell line (300-19) with G protein-coupled receptors that couple exclusively to Gαq (the m3 muscarinic receptor), Gαi (the κ-opioid receptor), and Gαs (the β-adrenergic receptor), as well as the human thrombin receptor (PAR-1) and the C-C chemokine receptor 2B. Cells expressing receptors that coupled to Gαi, but not to Gαq or Gαs, migrated in response to a concentration gradient of the appropriate agonist. Overexpression of Gα transducin, which binds to and inactivates free Gβγ dimers, completely blocked chemotaxis although having little or no effect on intracellular calcium mobilization or other measures of cell signaling. The identification of Gβγ dimers as a crucial intermediate in the chemotaxis signaling pathway provides further evidence that chemotaxis of mammalian cells has important similarities to polarized responses in yeast. We conclude that chemotaxis is dependent on activation of Gαi and the release of Gβγ dimers, and that Gαi-coupled receptors not traditionally associated with chemotaxis can mediate directed migration when they are expressed in hematopoietic cells.
Chemotaxis is crucial for leukocytes to migrate to sites of inflammation and infection, and a large number of chemotactic peptides (1, 2) and lipids (3) have been demonstrated to be chemoattractants. The most recent addition to the list of leukocyte chemoattractants is the rapidly growing family of proteins known as the chemokines (chemotactic cytokines) (for reviews, see refs. 4–8). The chemokines can be subdivided into two major families, based on the positions of the first two of four conserved cysteines and on the quaternary structure of chemokine homodimers (9, 10). Two chemokines have also recently been described: lymphotactin (11), which lacks the first and third cysteines, and fractalkine (12), which is displayed at the tip of a mucin stalk on the cell surface. Monocyte chemoattractant protein 1 (MCP-1) is a member of the C-C or β-chemokine family, in which the first two cysteines are adjacent, and is a potent chemoattractant for monocytes, basophils, and memory T cells. The receptors for all known leukocyte chemoattractants, including the chemokines, are members of the seven-transmembrane domain superfamily and couple to a variety of G protein α subunits, including Gαi, Gαq, and Gα16 (13, 14). The signal transduction pathways that lead to chemotaxis, however, have not yet been identified.
Unlike freshly isolated leukocytes, immortalized cell lines do not migrate well in chemotaxis assays, even when transfected with receptors for potent chemoattractants (15), and this has hampered efforts to elucidate the molecular mechanisms of chemotaxis. To investigate this question, we used a lymphocyte cell line (300-19) and a transwell assay system recently shown to produce robust chemotaxis (16, 17). Our results support the hypothesis that activation of Gαi-coupled receptors and the subsequent release of Gβγ dimers is required to initiate signal transduction leading to directed cell migration.
MATERIALS AND METHODS
Reagents.
MCP-1 was obtained from R & D Systems. RPMI 1640 medium and 2-mercaptoethanol were from Life Technology (Grand Island, NY). M1 antibody and antibody against extracellular signal-regulated kinase 2 (ERK2) were obtained from Kodak (IBI/Kodak, New Haven, CT) and Santa Cruz Biotechnology, respectively. Fetal calf serum was from HyClone Laboratories (Logan, UT). Pertussis toxin (PTX) was from List Biological Laboratories (Campbell, CA). U50488 was from Research Biochemicals (Natick, MA). [γ-32P]ATP was purchased from Amersham. All other reagents were obtained from Sigma.
DNA Constructs.
Epitope-tagged pCMV-1-CCR2B was prepared as described (18, 19). Epitope-tagged κ-opioid receptor (20) and the Gα transducin (Gαt) construct (21) were gifts of Bruce Conklin (Gladstone Institute). The m3 muscarinic receptor construct (22) was a gift from Jürgen Wess (National Institutes of Health). The epitope-tagged thrombin receptor construct [protease-activated receptor 1 (PAR-1)] (23) was from Shaun Coughlin (University of California, San Francisco). The β2-adrenergic receptor construct was from Mark Von Zastro (University of California, San Francisco).
Cell Culture and Transfection.
300-19 pre-B cells (24) were a generous gift of G. La Rousa (LeukoSite, Cambridge, MA) and were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), penicillin (100 units/ml), glutamine (2 mM), and 2-mercaptoethanol (55 μM). The cells were transfected by electroporation as described (17). Briefly, 2 × 106 cells in 400 μl of medium were mixed with 30 μg of each cDNA and 1.5 μg of a plasmid that conferred resistance to neomycin (pSV2-neo) and maintained at 4°C for 15 min. Electroporation was performed by using 250 V and 960 microfaradays. After an overnight incubation, G418 (800 μg/ml) was added to select for successfully transfected clones. Cells expressing both C-C chemokine receptor 2B (CCR2B) and transducin were prepared by transfecting Gαt in a plasmid containing a hygromycin-resistance gene into 300-19 cells stably expressing CCR2B. Double transfectants were obtained by maintaining the cells in both G418 (800 μg/ml) and hygromycin (100 μg/ml).
Fluorescence-Activated Cell Sorter (FACS) Analysis.
G418-resistant cells were analyzed for cell-surface expression of receptors with a FACS. Approximately 3 × 106 harvested cells were incubated at 37°C for 30 min in medium containing a phycoerythrin-conjugated M1 antibody. Unbound antibody was removed by washing with PBS, and the cells were resuspended in PBS plus 20 μg/ml propidium iodide.
Ca2+ Measurement.
Agonist-dependent increases in cytoplasmic Ca2+ were determined in transfected 300-19 cells as described (25).
ERK Assay.
ERK activation was determined as described (26). Briefly, 1 × 107 300-19 cells stably transfected with CCR2B were maintained in serum-free medium for 24 h and then activated by the addition of 10 nM MCP-1 for 5 min. After two washes with ice-cold PBS containing 1 mM sodium orthovanadate, the cells were lysed in 500 μl of ice-cold buffer (20 mM Hepes, pH 7.4/50 mM NaCl/1% Triton X-100/20 μM leupeptin/1 mM phenylmethylsulfonyl fluoride/10 μg/ml aprotinin/1 mM sodium orthovanadate/50 mM sodium fluoride) and centrifuged at 13,000 × g for 15 min. Equal amounts of cell lysates were then incubated with an anti-ERK2 antibody (Santa Cruz Biotechnology) for 2 h at 4°C, followed by the addition of protein A-agarose beads. The samples were washed three times with lysis buffer and twice with washing buffer (25 mM Tris, pH 7.4/25 mM β-glycerophosphate/1 mM sodium orthovanadate) and then incubated for 30 min at 25°C in 50 μl of kinase buffer (25 mM Hepes, pH 7.4/10 mM magnesium acetate/2 mM DTT/2 mM EGTA/200 μM sodium orthovanadate/50 μM ATP/250 μg/ml myelin basic protein/2 μM protein kinase A inhibitor/2 μCi [γ-32P]ATP; 1 Ci = 37 GBq). Laemmli sample buffer was then added (27), followed by electrophoresis and visualization of the phosphoproteins by autoradiography. Quantitation was performed by using the AMBIS system as described (28).
Chemotaxis Assay.
The migration of 300-19 cell lines was determined by using a modification of the method of Campbell et al. (16, 17). Briefly, 300-19 cells were resuspended in RPMI 1640 medium plus BSA (1 mg/ml). The cell density was adjusted to 5 × 106 cells/ml, 100 μl of the cell suspension was added to the top chamber of a 24-transwell apparatus (6.5 mm diameter, 5 μm pore size; Corning Costar), and the appropriate agonists were added to the lower wells. The plates were incubated for 3 h at 37°C in an atmosphere containing 5% CO2. Cells that passed through the membrane were collected from the lower well and counted in a FACScan (Becton Dickinson). The chemotactic index or ratio of cells in the lower chamber in the presence versus the absence of chemoattractant, was determined for each concentration of MCP-1. Cell numbers were also determined with a Coulter counter and found to be in excellent agreement with the results of the FACS analysis.
RESULTS
Intracellular Calcium Mobilization.
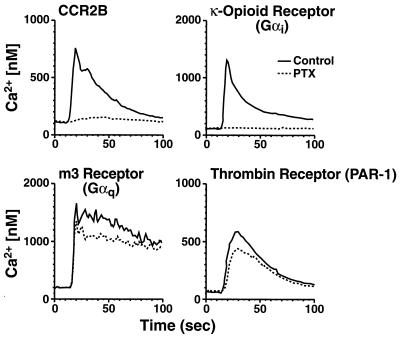
We have previously shown that the MCP-1 receptor CCR2B couples to multiple G proteins, including Gαi and Gαq, to initiate cellular activation (14). To identify G proteins specifically involved in mediating chemotaxis, we stably transfected a murine pre-B cell line (300-19) with receptors that couple to Gαi (the κ-opioid receptor), Gαq (the m3 muscarinic receptor), or Gαq and Gαi (the PAR-1 thrombin receptor and CCR2B). Expression of each receptor at the cell surface was verified by flow cytometry (data not shown), and functionality was assessed by measuring the mobilization of intracellular calcium in response to appropriate agonists. Each receptor demonstrated a robust, agonist-dependent calcium response (Fig. 1). Pretreatment with PTX blocked the response of CCR2B-transfected cells, indicating that MCP-1 signals almost exclusively through Gαi in 300-19 cells. PTX also blocked the response of cells expressing the κ-opioid receptor, as expected, but it did not block the thrombin-induced calcium flux in PAR-1-transfected cells, indicating that, in these cells, PAR-1 is largely Gαq-coupled. As expected, PTX had no effect on the Gαq-coupled m3 muscarinic receptor.
Figure 1.
Agonist-dependent calcium mobilization in 300-19 cells expressing Gαi- and Gαq-coupled receptors. Stably transfected 300-19 cells were incubated with or without 100 ng/ml of PTX for 24 h and loaded with Indo-1 AM. Intracellular calcium levels were measured as described. Shown are calcium fluxes in response to 100 nM of MCP-1 (CCR2B), 100 nM of U50488 (κ-opioid receptor), 200 μM of carbachol (m3 receptor), and 10 nM of thrombin (PAR-1 thrombin receptor) from a representative experiment (n = 3). The agonists used did not induce calcium fluxes in the absence of the appropriate cognate receptors.
Chemotaxis of G Protein-Coupled Receptors.
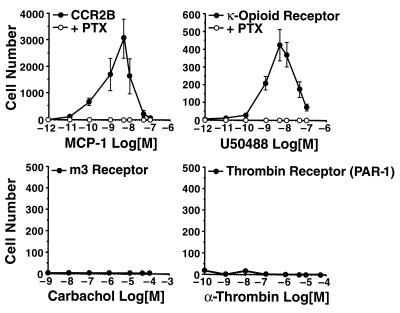
We next examined the ability of each cell line to undergo chemotaxis in response to the appropriate agonist. In the presence of MCP-1, 300-19 cells transfected with CCR2B demonstrated a typical bell-shaped pattern of chemotactic responses (Fig. 2). The maximal response, a chemotactic index of over 3,000, was seen at an MCP-1 concentration of 5 nM and was completely blocked by pretreatment of the cells with PTX. Cells transfected with the κ-opioid receptor also demonstrated chemotaxis in response to the synthetic agonist U50488, although with a significantly lower chemotactic index than CCR2B-transfected cells. This response was also blocked by PTX. In contrast, cells expressing either the Gαq-coupled m3 muscarinic receptor or the thrombin receptor failed to mediate chemotaxis, despite the robust intracellular Ca2+ mobilization induced by their respective agonists. The Gαs-coupled β2-adrenergic receptor also failed to mediate chemotaxis in the 300-19 cells (data not shown). These data indicate that Gαi-coupled receptors, but not Gαq- or Gαs-coupled receptors, initiate signal transduction that culminates in chemotaxis, and suggest that calcium mobilization alone is not sufficient to induce directed migration.
Figure 2.
Chemotaxis in 300-19 cells expressing Gαi- and Gαq-coupled receptors. Chemotaxis was analyzed by using transwells as described. Cells transfected with CCR2B and the κ-opioid receptor were incubated with or without 100 ng/ml of PTX for 24 h before the chemotaxis assays. The data are the means ± SE of four independent experiments.
Effect of Transducin Overexpression on Chemotaxis in Cells Expressing CCR2B.
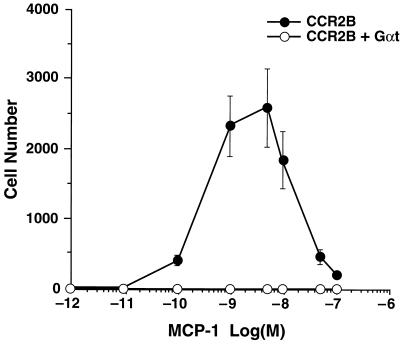
To determine whether Gβγ was critically involved in mediating CCR2B-mediated chemotaxis, we took advantage of the high affinity with which Gαt binds to and inactivates βγ dimers (21). Transducin was overexpressed in 300-19 cells stably transfected with CCR2B, and protein expression was verified by Western blot analysis (data not shown). As expected, cells expressing only CCR2B showed a robust chemotactic response to MCP-1 (Fig. 3). In contrast, virtually no chemotaxis was observed in cells expressing both CCR2B and transducin, suggesting that the βγ subunit of Gαi was required for directed migration.
Figure 3.
Inhibition of chemotaxis by overexpression of Gαt. Chemotaxis was induced in cells stably expressing CCR2B or CCR2B + Gαt. Data are the means ± SE of four independent experiments.
Effect of Transducin Overexpression on Ca2+ Mobilization and ERK Activation.
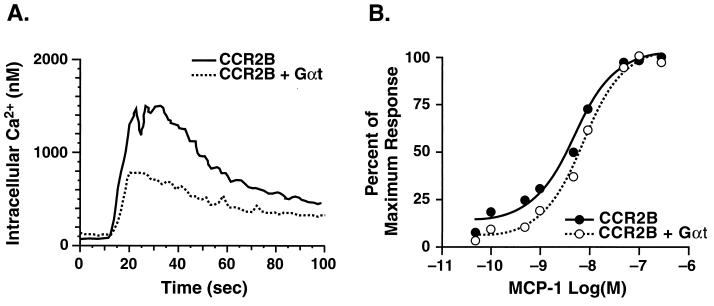
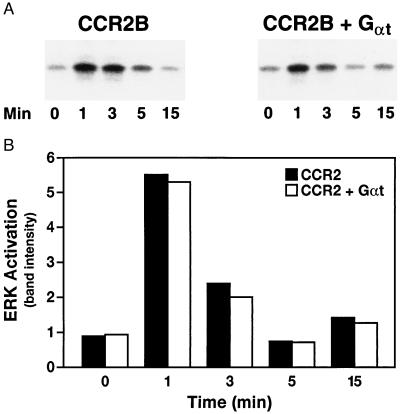
In contrast to its effects on chemotaxis, transducin only partially blocked MCP-1-induced calcium mobilization. As shown in Fig. 4, overexpression of Gαt reduced the maximal calcium response by ≈50% (Fig. 4A), but had little or no effect on the dose-response curve (Fig. 4B). To further characterize the effects of transducin, we measured MCP-1-dependent ERK activation in these cells. The time course and extent of ERK activation was unaffected by overexpression of transducin (Fig. 5).
Figure 4.
Ca2+ mobilization in 300-19 cells expressing CCR2B and Gαt. Stably transfected 300-19 cells were loaded with Indo-1 AM, and intracellular calcium levels in response to 100 nM MCP-1 (A) or to the indicated concentrations of MCP-1 (B) were measured as described in Fig. 1. Data are representative of three independent experiments.
Figure 5.
ERK activation in 300-19 cells expressing CCR2B and Gαt. Stably transfected 300-19 cells were maintained in serum-free medium for 24 h and incubated with 10 nM MCP-1 for the indicated times; lysates were assayed for ERK activation (A) as described. Quantitation is presented in B. Data are representative of three independent experiments.
DISCUSSION
By using cotransfection in COS-7 cells, we recently found that CCR2, a receptor for the C-C chemokine MCP-1, coupled to Gαi, Gα16, and Gαq (14). Receptors for the chemotactic peptides C5a, interleukin 8, MIP-1α, and RANTES have also been shown to signal via multiple G proteins in the Gαi and Gαq families (13, 14). To elucidate the G proteins critical for chemotaxis, we stably expressed CCR2 and prototypic Gαi- and Gαq-coupled receptors in 300-19 cells, an immortalized pre-B cell line (24). There are four major conclusions from the current studies. First, activation of Gαi-coupled receptors, but not Gαq- or Gαs-coupled receptors, leads to chemotaxis. Second, the large intracellular calcium flux typically seen in response to activation of G protein-coupled receptors is not sufficient to cause chemotaxis. Third, production of G protein βγ dimers is required for chemotaxis. Finally, when expressed in a hematopoietic cell line, Gαi-coupled receptors not found on leukocytes or traditionally associated with the directed migration of cells can mediate chemotaxis.
Analysis of the molecular mechanisms of chemotaxis has been hampered by the poor migration of cells transfected with chemokine or other chemotactic peptide receptors, relative to that of freshly isolated leukocytes (15). In this study, we used a pre-B cell line that, when transfected with chemokine receptors and examined in a transwell assay, gave a chemotactic index in excess of 3,000 (17). This robust response was completely blocked by pretreating the cells with PTX, as might be expected from its inhibitory effect on chemotaxis of blood leukocytes (29–31). More surprisingly, cells transfected with Gαq-coupled receptors showed no chemotactic response, even though activation of Gαi- and Gαq-coupled receptors induced very similar rises in intracellular calcium. A clear result of this study, therefore, is that agonist-induced elevation of intracellular calcium is not sufficient to induce chemotaxis.
To determine whether activation of Gαi by a nonchemokine receptor would also lead to chemotaxis, we stably transfected 300-19 cells with the κ-opioid receptor, which couples exclusively to Gαi and is not ordinarily expressed in hematopoietic cells. We consistently noted a classic, biphasic migratory response of these cells in response to U50488, a small molecule agonist of the receptor. Checkerboard analysis demonstrated that this was chemotaxis and not chemokinesis. The magnitude of this response, however, was consistently lower than that of CCR2, suggesting that chemokine receptors may contribute more to chemotaxis than simply activating Gαi.
Activation of CCR2 or the κ-opioid receptor causes dissociation of the G protein heterotrimeric complex into activated Gαi and free βγ dimers. Inhibition of CCR2-mediated chemotaxis by coexpression of Gαt, which stoichiometrically binds to and inactivates βγ dimers (21), provided evidence that the βγ dimers were a necessary intermediate in the chemotactic signaling pathway. Coexpression of Gαt only partially blocked MCP-1-induced calcium mobilization and had no apparent effect on ERK activation, indicating that CCR2 remained functionally coupled to Gαi. Crespo et al. (32) overexpressed Gαt in COS-7 cells transfected with the m1 and m2 muscarinic receptors and found inhibition of ERK activation. It is likely that the relatively low level of Gαt expression in our cells accounts for the failure to block ERK activation or calcium mobilization. Thus, these data demonstrate that modest overexpression of Gαt completely blocks chemotaxis, without abolishing the ability of the cell to support agonist-dependent signal transduction.
Results that are complementary and completely consistent with these data have recently been obtained by Neptune et al. (33). Specifically, these investigators found that transfection with the Gαi-coupled δ- or μ-opioid receptors, or the D2 dopamine receptor, conferred on HEK-293 cells the ability to undergo chemotaxis to the appropriate agonists. In contrast, the Gq-coupled m3 muscarinic receptor and the Gs-coupled β2-adrenergic receptor failed to promote chemotaxis. This group further found that sequestration of free Gβγ dimers by overexpression of either transducin or a fragment of the β-adrenergic receptor kinase [βark-495-689] (21) blocked agonist-dependent chemotaxis in HEK-293 cells transfected with the interleukin 8 receptor. Taken together with the data in this paper, these results support the hypothesis that chemotaxis is a consequence of activation of Gαi and that free Gβγ dimers are necessary for the chemotactic response.
We were surprised that the α-thrombin receptor, PAR-1, failed to support chemotaxis in 300-19 cells. Thrombin mediates the chemotaxis of monocytes/macrophages (34, 35) as well as fibroblasts (36), but the studies that demonstrate this have been difficult to interpret mechanistically because catalytically inactive forms of thrombin, which fail to activate PAR-1, are also chemotactic (34, 35). Although PAR-1 couples to both Gαi and Gαq in fibroblasts (37), we found that in 300-19 cells, it signaled almost exclusively through Gαq. The G protein coupling of PAR-1 is thus highly cell-type dependent, and it is likely that its failure to mediate chemotaxis in 300-19 cells was caused by its inability to activate Gαi in these cells. It is also possible, however, that the very recently reported receptor PAR-3 (38) is the thrombin receptor that mediates chemotaxis in monocytes/macrophages. Experiments designed to examine this possibility are currently in progress.
Because activation of Gαq- as well as Gαi-coupled receptors leads to liberation of βγ dimers, it is interesting to speculate why only Gαi receptors initiate chemotaxis. It is possible that different isoforms of β and γ subunits associate with Gαi versus Gαq receptors, though there are few data to support this notion. Alternatively, the spatial localization of the βγ dimers, which are retained at the plasma membrane through prenylation (39, 40), may differ between Gαi- and Gαq/Gαs-coupled receptors, and this may be an important factor in initiating chemotaxis, and perhaps changes in the local calcium concentration. The downstream target(s) of the βγ dimers are not yet known, but given the dramatic cytoskeletal changes associated with chemotaxis, involvement of the Rac/CDC42Hs family of small GTPases seems likely.
There are interesting similarities between the chemotaxis of leukocytes and the polarized responses in yeast (reviewed in ref. 41). In Saccharomyces cerevisiae cells, secretion of pheromones causes the directional extension of psuedopods (shmoos) toward the mating partner, a process that involves cytoskeletal reorganization (42). Pheromones bind to and activate G protein-coupled receptors, and in yeast, the primary signaling molecule is the Gβγ dimer. The release of free βγ dimers initiates shmoo formation. The immediate targets of βγ dimers are not yet clear, but several interesting candidates have been identified in yeast (43, 44). If the directed migration of mammalian cells is an evolutionary adaptation of shmoo formation, then elucidation of the mechanisms of chemotaxis may be greatly aided by the clues from the study of polarized responses in yeast.
Acknowledgments
We thank Drs. Henry Bourne and Enid Neptune for helpful scientific discussions and for sharing unpublished data with us. We thank Drs. Bruce Conklin and Robert Pitas for a careful reading of the manuscript, John Carroll and Brian Clark for preparation of graphics, Stephen Ordway and Gary Howard for editorial assistance, and Angela Chen for manuscript preparation. This work was funded in part by National Institutes of Health Grant HL52773 (I.F.C.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CCR, C-C chemokine receptor; ERK, extracellular signal-regulated kinase; FACS, fluorescence-activated cell sorter; Gαt, Gα transducin; MCP-1, monocyte chemoattractant protein 1; PAR-1, protease-activated receptor 1; PTX, pertussis toxin.
References
- 1.Hugli T E, Gerard C, Kawahara M, Scheetz M E, II, Barton R, Briggs S, Koppel G, Russell S. Mol Cell Biochem. 1981;41:59–66. doi: 10.1007/BF00225297. [DOI] [PubMed] [Google Scholar]
- 2.Schiffmann E, Corcoran B A, Wahl S M. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. Nature (London) 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 5.Hedrick J A, Zlotnik A. Curr Opin Immunol. 1996;8:343–347. doi: 10.1016/s0952-7915(96)80123-3. [DOI] [PubMed] [Google Scholar]
- 6.Murphy P M. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 7.Schall T J. In: The Cytokine Handbook. Thomson A W, editor. London: Academic; 1994. pp. 419–460. [Google Scholar]
- 8.Gerard C, Gerard N P. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 9.Lodi P J, Garrett D S, Kuszewski J, Tsang M L-S, Weatherbee J A, Leonard W J, Gronenborn A M, Clore G M. Science. 1994;263:1762–1767. doi: 10.1126/science.8134838. [DOI] [PubMed] [Google Scholar]
- 10.Clore G M, Gronenborn A M. FASEB J. 1995;9:57–62. doi: 10.1096/fasebj.9.1.7821760. [DOI] [PubMed] [Google Scholar]
- 11.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, Zlotnik A. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 12.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. Nature (London) 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 13.Kuang Y, Wu Y, Jiang H, Wu D. J Biol Chem. 1996;271:3975–3978. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- 14.Arai H, Charo I F. J Biol Chem. 1996;271:21814–21819. doi: 10.1074/jbc.271.36.21814. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Baruch A, Bengali K M, Biragyn A, Johnston J J, Wang J-M, Kim J, Chuntharapai A, Michiel D F, Oppenheim J J, Kelvin D J. J Biol Chem. 1995;270:9121–9128. doi: 10.1074/jbc.270.16.9121. [DOI] [PubMed] [Google Scholar]
- 16.Campbell J J, Qin S, Bacon K B, Mackay C R, Butcher E C. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosling J, Monteclaro F S, Atchison R E, Arai H, Tsou C-L, Goldsmith M A, Charo I F. Proc Natl Acad Sci USA. 1997;94:5061–5066. doi: 10.1073/pnas.94.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson S, Davis D L, Dahlbäck H, Jörnvall H, Russell D W. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 19.Arai H, Monteclaro F S, Tsou C-L, Franci C, Charo I F. J Biol Chem. 1997;272:25037–25042. doi: 10.1074/jbc.272.40.25037. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Chen C, Xue J-C, Kunapuli S, DeRiel J K, Liu-Chen L-Y. Life Sci. 1995;56:PL201–PL207. doi: 10.1016/0024-3205(94)00507-o. [DOI] [PubMed] [Google Scholar]
- 21.Lustig K D, Conklin B R, Herzmark P, Taussig R, Bourne H R. J Biol Chem. 1993;268:13900–13905. [PubMed] [Google Scholar]
- 22.Wess J, Bonner T I, Brann M R. Mol Pharmacol. 1990;38:872–877. [PubMed] [Google Scholar]
- 23.Vu T-K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 24.Reth M G, Ammirati P, Jackson S, Alt F W. Nature (London) 1985;317:353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- 25.Myers S J, Wong L M, Charo I F. J Biol Chem. 1995;270:5786–5792. doi: 10.1074/jbc.270.11.5786. [DOI] [PubMed] [Google Scholar]
- 26.Arai H, Escobedo J A. Mol Pharmacol. 1996;50:522–528. [PubMed] [Google Scholar]
- 27.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Yamanaka S, Balestra M E, Ferrell L D, Fan J, Arnold K S, Taylor S, Taylor J M, Innerarity T L. Proc Natl Acad Sci USA. 1995;92:8483–8487. doi: 10.1073/pnas.92.18.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sozzani S, Luini W, Molino M, Jílek P, Bottazzi B, Cerletti C, Matsushima K, Mantovani A. J Immunol. 1991;147:2215–2221. [PubMed] [Google Scholar]
- 30.Thelen M, Peveri P, Kernen P, Von Tscharner V, Walz A, Baggiolini M. FASEB J. 1988;2:2702–2706. [PubMed] [Google Scholar]
- 31.Bischoff S C, Krieger M, Brunner T, Rot A, von Tscharner V, Baggiolini M, Dahinden C A. Eur J Immunol. 1993;23:761–767. doi: 10.1002/eji.1830230329. [DOI] [PubMed] [Google Scholar]
- 32.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 33.Neptune E R, Bourne H R. Proc Natl Acad Sci USA. 1997;94:14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bar-Shavit R, Hruska K A, Kahn A J, Wilner G D. J Cell Physiol. 1987;131:255–261. doi: 10.1002/jcp.1041310216. [DOI] [PubMed] [Google Scholar]
- 35.Crago A M, Wu H-F, Hoffman M, Church F C. Exp Cell Res. 1995;219:650–656. doi: 10.1006/excr.1995.1275. [DOI] [PubMed] [Google Scholar]
- 36.Dawes K E, Gray A J, Laurent G J. Eur J Cell Biol. 1993;61:126–130. [PubMed] [Google Scholar]
- 37.Hung D T, Wong Y H, Vu T-K H, Coughlin S R. J Biol Chem. 1992;267:20831–20834. [PubMed] [Google Scholar]
- 38.Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 39.Mumby S M, Casey P J, Gilman A G, Gutowski S, Sternweis P C. Proc Natl Acad Sci USA. 1990;87:5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonds W F, Butrynski J E, Gautam N, Unson C G, Spiegel A M. J Biol Chem. 1991;266:5363–5366. [PubMed] [Google Scholar]
- 41.Chenevert J. Mol Biol Cell. 1994;5:1169–1175. doi: 10.1091/mbc.5.11.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes G, Drubin D G, Stearns T. Curr Opin Cell Biol. 1990;2:109–115. doi: 10.1016/s0955-0674(05)80040-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Z-S, Leung T, Manser E, Lim L. Mol Cell Biol. 1995;15:5246–5257. doi: 10.1128/mcb.15.10.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akada R, Kallal L, Johnson D I, Kurjan J. Genetics. 1996;143:103–117. doi: 10.1093/genetics/143.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]