Abstract
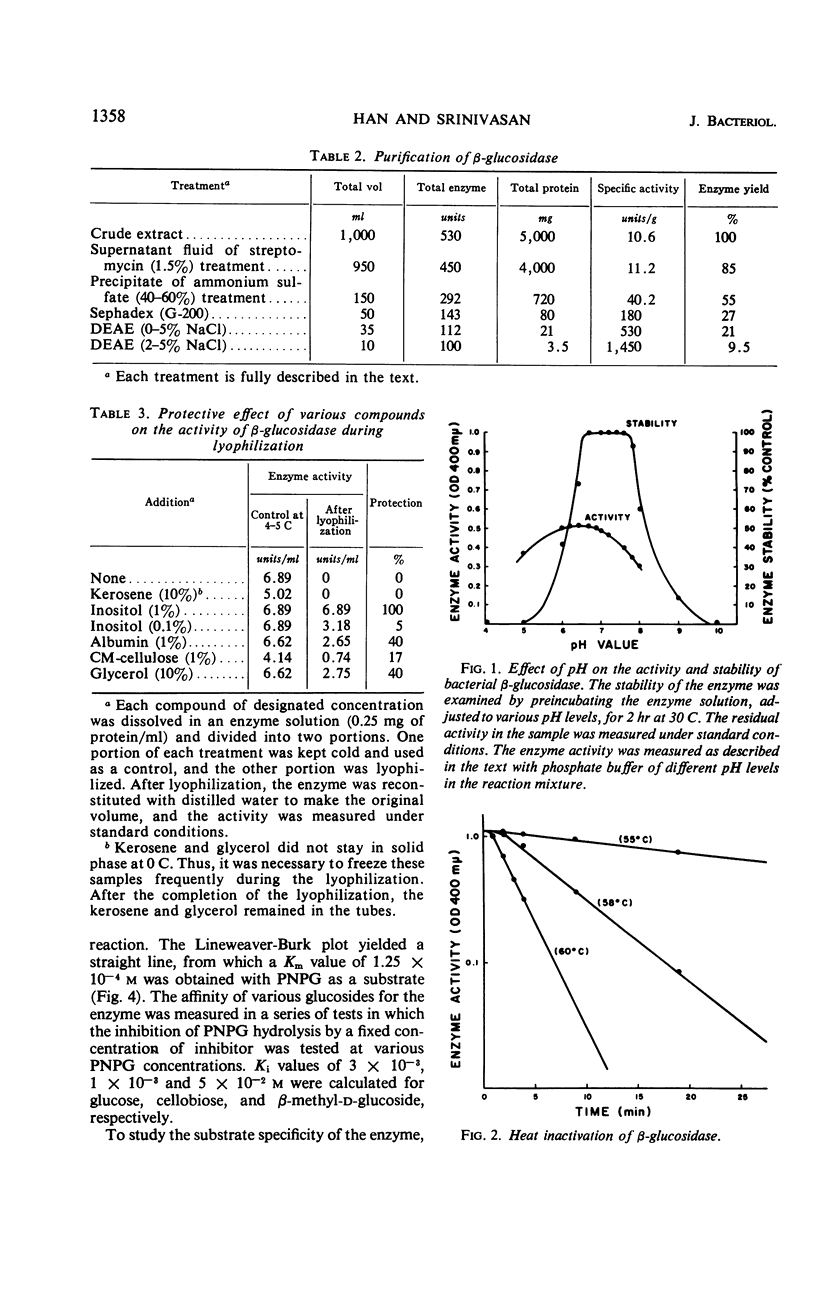
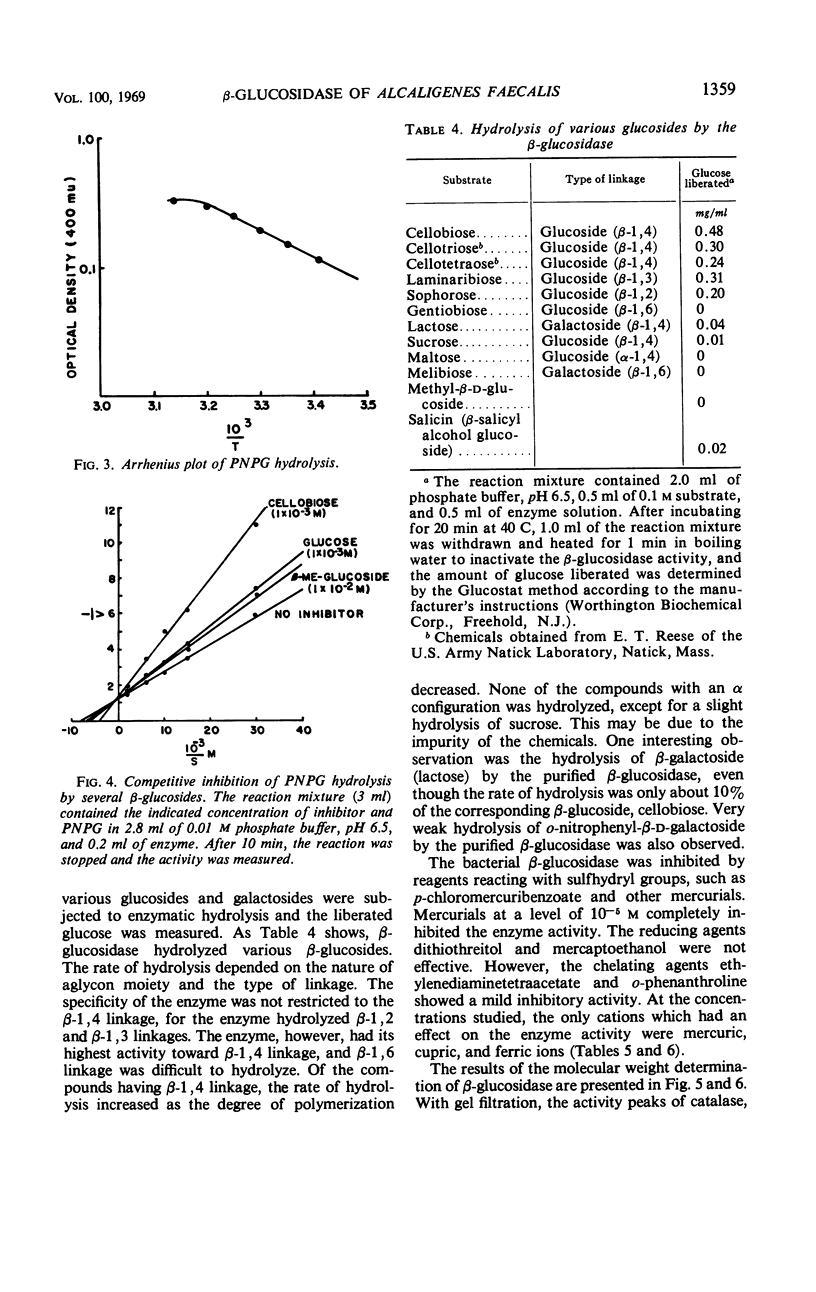
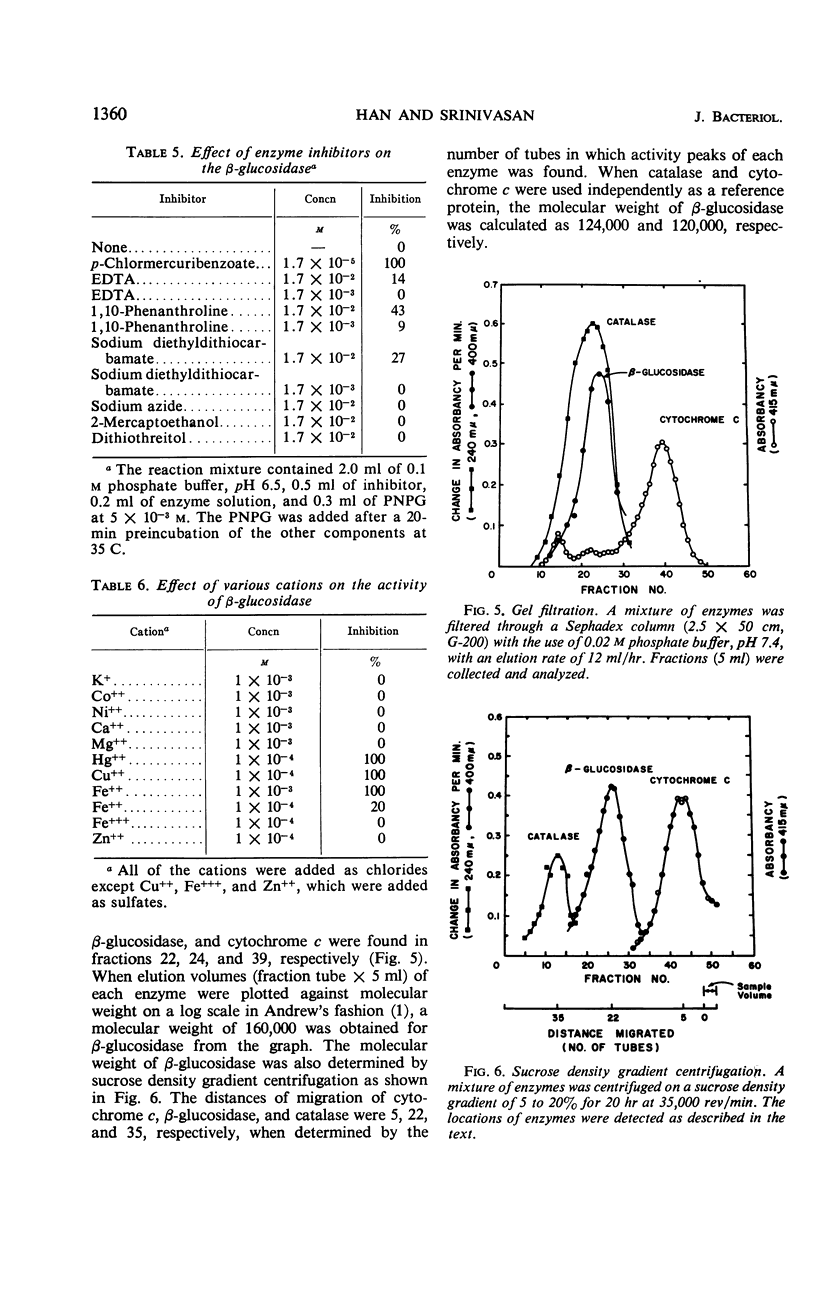
A cellobiose-utilizing bacterium isolated from sugar cane bagasse and identified as a strain of Alcaligenes faecalis (ATCC 21400) produced an inducible β-glucoside-splitting enzyme. The enzyme was purified by a series of streptomycin and ammonium sulfate fractionations and by Sephadex and diethylaminoethyl column chromatography. The final preparation was purified 130-fold, with a recovery of about 10% of the initial enzyme activity. The enzyme had a wide pH range, with optimal activity at pH 6.0 to 7.0. The enzyme was stable in solution at pH 6.5 to 7.8 when kept at 30 C for 2 hr, but it was destroyed by temperatures above 55 C. At 58 and 60 C, the time required to inactivate 90% of the initial activity was 16 and 6.5 min, respectively. An activation energy of 9,500 cal/mole and a Km of 1.25 × 10−4m were obtained by using p-nitrophenyl β-glucoside as a substrate. The Ki value and hydrolysis of cellobiose by the enzyme indicated a high affinity of the enzyme for the cellobiose. The enzyme had its specificity on β-glucosidic linkage and the rate of hydrolisis of glucosides depended upon the nature of the aglycon moiety. The inactivation studies showed the presence of sulfhydryl groups in the enzyme. The activity of the enzyme was easily destroyed by the Cu++ and Hg++ ions. The Michaelis-Menton relationship and the rate of heat inactivation indicated the presence of one type of noninteracting active site in the bacterial β-glucosidase. Molecular weight of the enzyme was estimated by gel filtration (Sephadex G-200) and sucrose density gradient, and a value of 120,000 to 160,000 was obtained.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONCHIE J. Beta-Glucosidase from rumen liquor; preparation, assay and kinetics of action. Biochem J. 1954 Dec;58(4):552–560. doi: 10.1042/bj0580552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOK E. M., STONE B. A. The enzymic hydrolysis of beta-glucosides. Biochem J. 1957 Jan;65(1):1–12. doi: 10.1042/bj0650001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Pig intestinal beta-glucosidase activities. I. Relation to beta-galactosidase (lactase). Biochim Biophys Acta. 1961 Jun 10;50:55–61. doi: 10.1016/0006-3002(61)91059-9. [DOI] [PubMed] [Google Scholar]
- DUERKSEN J. D., HALVORSON H. Purification and properties of an inducible beta-glucosidase of yeast. J Biol Chem. 1958 Nov;233(5):1113–1120. [PubMed] [Google Scholar]
- DUERKSEN J. D., HALVORSON H. The specificity of induction of beta-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1959 Nov;36:47–55. doi: 10.1016/0006-3002(59)90068-x. [DOI] [PubMed] [Google Scholar]
- Fleming L. W., Duerksen J. D. Evidence for multiple molecular forms of yeast beta-glucosidase in a hybrid yeast. J Bacteriol. 1967 Jan;93(1):142–150. doi: 10.1128/jb.93.1.142-150.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L. W., Duerksen J. D. Purification and characterization of yeast beta-glucosidases. J Bacteriol. 1967 Jan;93(1):135–141. doi: 10.1128/jb.93.1.135-141.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYWORTH R., WALKER P. G. Almond-emulsin beta-D-glucosidase and beta-D-galactosidase. Biochem J. 1962 May;83:331–335. doi: 10.1042/bj0830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU A. S., EPSTEIN R., HALVORSON H. O., BOCK R. M. Yeast beta-glucosidase: comparison of the physical-chemical properties of purified constitutive and inducible enzyme. Arch Biochem Biophys. 1960 Dec;91:210–218. doi: 10.1016/0003-9861(60)90492-6. [DOI] [PubMed] [Google Scholar]
- Han Y. W., Srinivasan V. R. Isolation and characterization of a cellulose-utilizing bacterium. Appl Microbiol. 1968 Aug;16(8):1140–1145. doi: 10.1128/am.16.8.1140-1145.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING K. W., SMIBERT R. M. Distinctive properties of beta-glucosidases and related enzymes derived from a commercial Aspergillus niger cellulase. Appl Microbiol. 1963 Jul;11:315–319. doi: 10.1128/am.11.4.315-319.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI L. H., KING K. W. Fractionation of beta-glucosidases and related extracellular enzymes from Aspergillus niger. Appl Microbiol. 1963 Jul;11:320–325. doi: 10.1128/am.11.4.320-325.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marchin G. L., Duerksen J. D. Comparison of the catalytic and immunological properties of beta-glucosidases from three strains of Saccharomyces lactis. J Bacteriol. 1969 Jan;97(1):237–243. doi: 10.1128/jb.97.1.237-243.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchin G. L., Duerksen J. D. Purification of beta-glucosidase from Saccharomyces lactis strain Y-123. J Bacteriol. 1968 Oct;96(4):1181–1186. doi: 10.1128/jb.96.4.1181-1186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchin G. L., Duerksen J. D. Purification of beta-glucosidase from Saccharomyces lactis strains Y-14 and Y-1057A. J Bacteriol. 1968 Oct;96(4):1187–1190. doi: 10.1128/jb.96.4.1187-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese E. T., Maguire A. H., Parrish F. W. Glucosidases and exo-glucanases. Can J Biochem. 1968 Jan;46(1):25–34. doi: 10.1139/o68-005. [DOI] [PubMed] [Google Scholar]
- SCHAFLER S., MINTZER L. Acquisition of lactose-fermenting properties by salmonellae. I. Interrelationship between the fermentation of cellobiose and lactose. J Bacteriol. 1959 Aug;78:159–163. doi: 10.1128/jb.78.2.159-163.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFLER S., MINTZER L., SCHAFLER C. Acquisition of lactose fermenting properties by salmonellae. II. Role of the medium. J Bacteriol. 1960 Feb;79:203–212. doi: 10.1128/jb.79.2.203-212.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



