Abstract
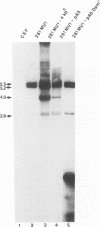
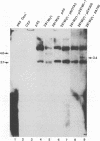
Previously, we isolated a replicon from a defective Marek's disease virus (MDV), analogous to defective herpes simplex viruses (amplicons). Defective viruses contain cis-acting elements required for DNA synthesis and virus propagation such as an origin of DNA replication and a packaging-cleavage signal site. In this report, the MDV replicon was utilized to locate an origin of MDV DNA replication. A comparison of MDV replicon sequences with other herpesvirus replication origin sequences revealed a 90-bp sequence containing 72% identity to the lytic origin (oris) of herpes simplex virus type 1. This 90-bp sequence displayed no similarity to betaherpesvirus or gammaherpesvirus replication origins. The 90-bp sequence is arranged as an imperfect palindrome centered around an A+T-rich region. This sequence also contains a 9-bp motif (5'CGTTCGCAC3') highly conserved in alphaherpesvirus replication origins. To test functionality of the 90-bp putative MDV replication origin, we conducted DpnI replication assays with subclones generated from the 4-kbp MDV replicon. A 700-bp MDV replicon subfragment containing the 90-bp putative MDV replication origin sequence is capable of replicating in chicken embryo fibroblast cells cotransfected with helper virus DNA. In conclusion, we identified a functional origin of DNA replication in MDV. Similarity of MDV origin sequences to those of alphaherpesviruses supports the current contention that MDV is more closely related to alphaherpesviruses than to gammaherpesviruses.
Full text
PDF
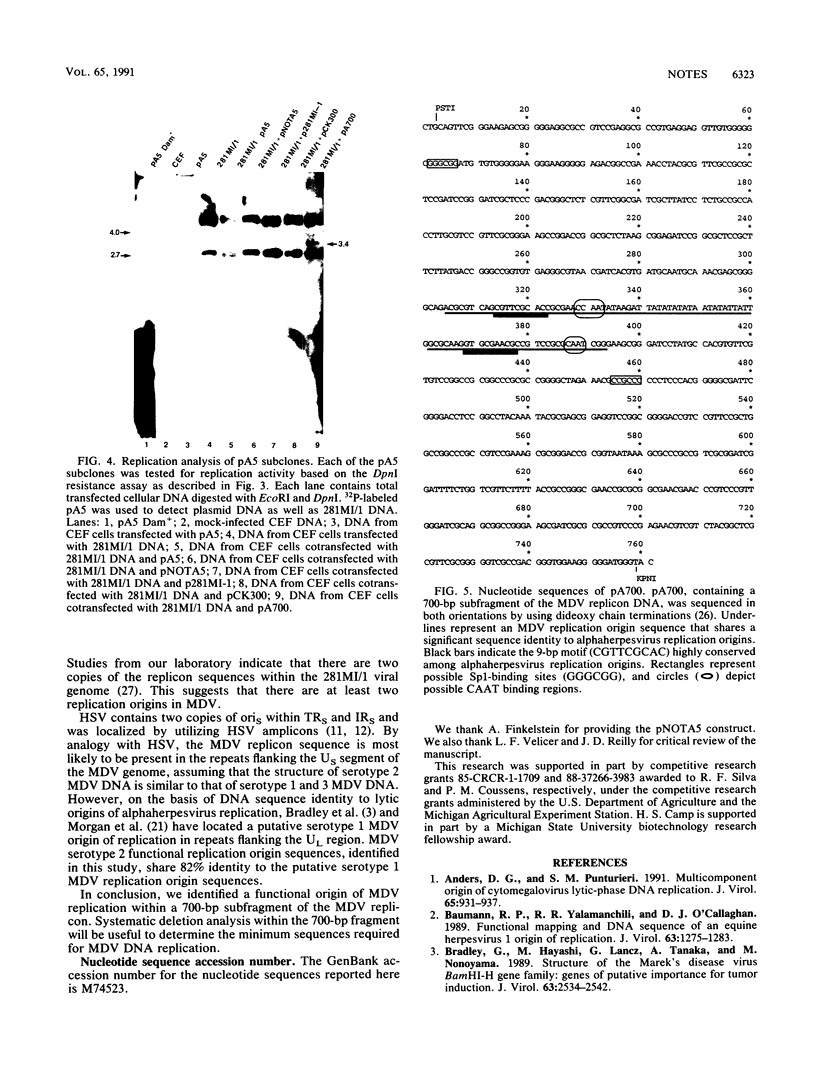

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Punturieri S. M. Multicomponent origin of cytomegalovirus lytic-phase DNA replication. J Virol. 1991 Feb;65(2):931–937. doi: 10.1128/jvi.65.2.931-937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann R. P., Yalamanchili V. R., O'Callaghan D. J. Functional mapping and DNA sequence of an equine herpesvirus 1 origin of replication. J Virol. 1989 Mar;63(3):1275–1283. doi: 10.1128/jvi.63.3.1275-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner R. C., Crute J. J., Dodson M. S., Lehman I. R. The herpes simplex virus 1 origin binding protein: a DNA helicase. J Biol Chem. 1991 Feb 5;266(4):2669–2674. [PubMed] [Google Scholar]
- Buckmaster A. E., Scott S. D., Sanderson M. J., Boursnell M. E., Ross N. L., Binns M. M. Gene sequence and mapping data from Marek's disease virus and herpesvirus of turkeys: implications for herpesvirus classification. J Gen Virol. 1988 Aug;69(Pt 8):2033–2042. doi: 10.1099/0022-1317-69-8-2033. [DOI] [PubMed] [Google Scholar]
- Budowle B., Baechtel F. S. Modifications to improve the effectiveness of restriction fragment length polymorphism typing. Appl Theor Electrophor. 1990;1(4):181–187. [PubMed] [Google Scholar]
- Carter J. K., Silva R. F. Cell culture amplification of a defective Marek's disease virus. Virus Genes. 1990 Sep;4(3):225–237. doi: 10.1007/BF00265632. [DOI] [PubMed] [Google Scholar]
- Deb S., Doelberg M. A 67-base-pair segment from the Ori-S region of herpes simplex virus type 1 encodes origin function. J Virol. 1988 Jul;62(7):2516–2519. doi: 10.1128/jvi.62.7.2516-2519.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P., Lehman I. R. Interaction of origin binding protein with an origin of replication of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1988 May;85(9):2959–2963. doi: 10.1073/pnas.85.9.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkeĺ N., Locker H., Batterson W., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA. VI. Defective DNA originates from the S component. J Virol. 1976 Nov;20(2):527–531. doi: 10.1128/jvi.20.2.527-531.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahn T. A., Schildkraut C. L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989 Aug 11;58(3):527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hernandez T. R., Dutch R. E., Lehman I. R., Gustafsson C., Elias P. Mutations in a herpes simplex virus type 1 origin that inhibit interaction with origin-binding protein also inhibit DNA replication. J Virol. 1991 Mar;65(3):1649–1652. doi: 10.1128/jvi.65.3.1649-1652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A., Tegtmeyer P. Characterization of major recognition sequences for a herpes simplex virus type 1 origin-binding protein. J Virol. 1988 Nov;62(11):4096–4103. doi: 10.1128/jvi.62.11.4096-4103.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus amplicon: effect of size on replication of constructed defective genomes containing eucaryotic DNA sequences. J Virol. 1984 Sep;51(3):595–603. doi: 10.1128/jvi.51.3.595-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshon D., Galloway D. A. Sequence and structural requirements of a herpes simplex viral DNA replication origin. Mol Cell Biol. 1988 Oct;8(10):4018–4027. doi: 10.1128/mcb.8.10.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Cantello J. L., Claessens J. A., Sondermeyer P. Inhibition of Marek's disease virus DNA transfection by a sequence containing an alphaherpesvirus origin of replication and flanking transcriptional regulatory elements. Avian Dis. 1991 Jan-Mar;35(1):70–81. [PubMed] [Google Scholar]
- Morgan R. W., Cantello J. L., McDermott C. H. Transfection of chicken embryo fibroblasts with Marek's disease virus DNA. Avian Dis. 1990 Apr-Jun;34(2):345–351. [PubMed] [Google Scholar]
- Polvino-Bodnar M., Orberg P. K., Schaffer P. A. Herpes simplex virus type 1 oriL is not required for virus replication or for the establishment and reactivation of latent infection in mice. J Virol. 1987 Nov;61(11):3528–3535. doi: 10.1128/jvi.61.11.3528-3535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. F., Lee L. F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease virus or turkey herpesvirus. Virology. 1984 Jul 30;136(2):307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Fadly A., Okazaki W. An enzyme-linked immunosorbent assay for detecting avian leukosis-sarcoma viruses. Avian Dis. 1979 Jul-Sep;23(3):698–707. [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci U S A. 1985 Feb;82(3):694–698. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Davison A. J. Identification of a varicella-zoster virus origin of DNA replication and its activation by herpes simplex virus type 1 gene products. J Gen Virol. 1986 Aug;67(Pt 8):1613–1623. doi: 10.1099/0022-1317-67-8-1613. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Localization of an origin of DNA replication within the TRS/IRS repeated region of the herpes simplex virus type 1 genome. EMBO J. 1982;1(7):863–867. doi: 10.1002/j.1460-2075.1982.tb01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983 Oct 30;130(2):427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Vlazny D. A., Frenkel N. Replication of herpes simplex virus DNA: localization of replication recognition signals within defective virus genomes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):742–746. doi: 10.1073/pnas.78.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Spadaro A., Schaffer J. E., Murray A. W., Maxam A. M., Schaffer P. A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985 May;5(5):930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter R. L. Characteristics of Marek's disease viruses isolated from vaccinated commercial chicken flocks: association of viral pathotype with lymphoma frequency. Avian Dis. 1983 Jan-Mar;27(1):113–132. [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]