Abstract
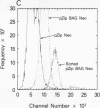
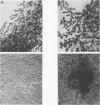
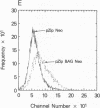
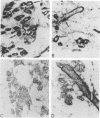
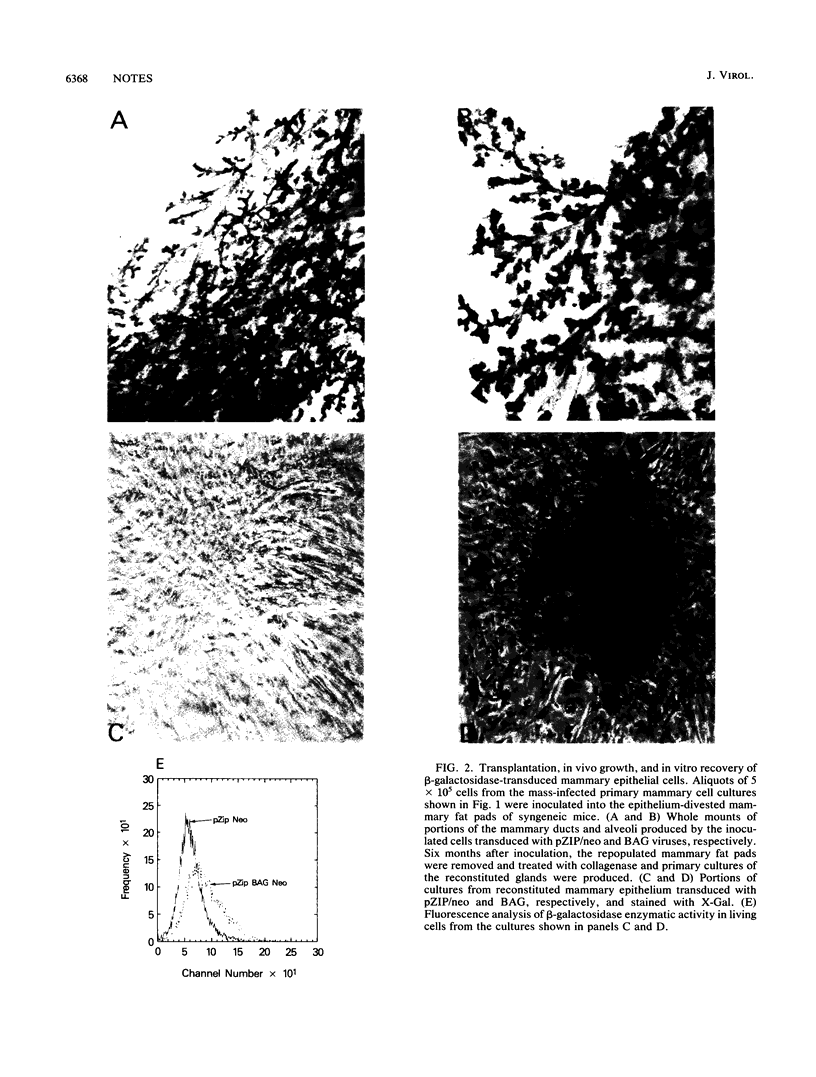
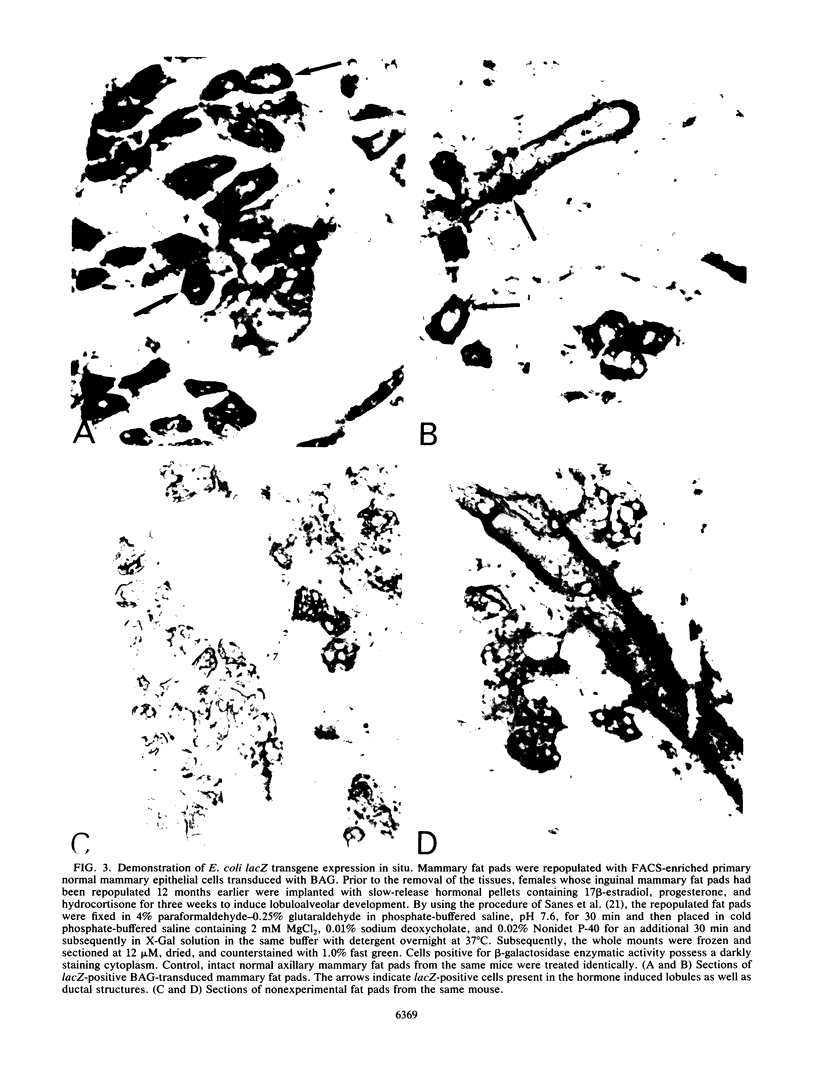
Nonimmortalized mouse mammary epithelial cells expressing Escherichia coli beta-galactosidase from a murine amphotropic packaged retroviral vector were injected into the epithelium-divested mammary fat pads of syngeneic mice. Mammary glands formed from the injected mammary epithelial cells contained ductal and lobular cells, both of which expressed beta-galactosidase when examined in situ more than 12 months later. These results indicate that stable recombinant gene expression can be achieved in vivo in the mammary gland without altering the growth properties of normal mammary epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Campbell G. Mutations in human breast cancer: an overview. J Natl Cancer Inst. 1989 Dec 6;81(23):1780–1786. doi: 10.1093/jnci/81.23.1780. [DOI] [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Daniel C. W., De Ome K. B., Young J. T., Blair P. B., Faulkin L. J., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A. 1968 Sep;61(1):53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C. W. Regulation of cell division in aging mouse mammary epithelium. Adv Exp Med Biol. 1975;61:1–19. doi: 10.1007/978-1-4615-9032-3_1. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Ward J. L., Bradbury J. M. Alteration of morphogenesis by the v-myc oncogene in transplants of mammary gland. Oncogene. 1988 Apr;2(4):407–412. [PubMed] [Google Scholar]
- Ehmann U. K., Guzman R. C., Osborn R. C., Young J. T., Cardiff R. D., Nandi S. Cultured mouse mammary epithelial cells: normal phenotype after implantation. J Natl Cancer Inst. 1987 Apr;78(4):751–757. [PubMed] [Google Scholar]
- Ehmann U. K., Peterson W. D., Jr, Misfeldt D. S. To grow mouse mammary epithelial cells in culture. J Cell Biol. 1984 Mar;98(3):1026–1032. doi: 10.1083/jcb.98.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAULKIN L. J., Jr, DEOME K. B. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst. 1960 Apr;24:953–969. [PubMed] [Google Scholar]
- HOSHINO K. Morphogenesis and growth potentiality of mammary glands in mice. I. Transplantability and growth potentiality of mammary tissue of virgin mice. J Natl Cancer Inst. 1962 Nov;29:835–851. [PubMed] [Google Scholar]
- HOSHINO K. REGENERATION AND GROWTH OF QUANTITATIVELY TRANSPLANTED MAMMARY GLANDS OF NORMAL FEMALE MICE. Anat Rec. 1964 Nov;150:221–235. doi: 10.1002/ar.1091500303. [DOI] [PubMed] [Google Scholar]
- Lin W. C., Pretlow T. P., Pretlow T. G., 2nd, Culp L. A. Bacterial lacZ gene as a highly sensitive marker to detect micrometastasis formation during tumor progression. Cancer Res. 1990 May 1;50(9):2808–2817. [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. V., Christoph G., Zeller R., Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol. 1990 Aug;10(8):4406–4411. doi: 10.1128/mcb.10.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci. 1988 May;90(Pt 1):173–183. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- Strange R., Aguilar-Cordova E., Young L. J., Billy H. T., Dandekar S., Cardiff R. D. Harvey-ras mediated neoplastic development in the mouse mammary gland. Oncogene. 1989 Mar;4(3):309–315. [PubMed] [Google Scholar]
- Zwiebel J. A., Freeman S. M., Kantoff P. W., Cornetta K., Ryan U. S., Anderson W. F. High-level recombinant gene expression in rabbit endothelial cells transduced by retroviral vectors. Science. 1989 Jan 13;243(4888):220–222. doi: 10.1126/science.2911735. [DOI] [PubMed] [Google Scholar]