Abstract
Immunological functions were analyzed in mice lacking granulocyte/macrophage colony-stimulating factor (GM-CSF). The response of splenic T cells to allo-antigens, anti-mouse CD3 mAb, interleukin 2 (IL-2), or concanavalin A was comparable in GM-CSF +/+ and GM-CSF −/− mice. To investigate the responses of CD8+ and CD4+ T cells against exogenous antigens, mice were immunized with ovalbumin peptide or with keyhole limpet hemocyanin (KLH). Cytotoxic CD8+ T cells with specificity for ovalbumin peptide could not be induced in GM-CSF −/− mice. After immunization with KLH, there was a delay in IgG generation, particularly IgG2a, in GM-CSF −/− mice. Purified CD4+ T cells from GM-CSF −/− mice immunized with KLH showed impaired proliferative responses and produced low amounts of interferon-γ (IFN-γ) and IL-4 when KLH-pulsed B cells or spleen cells were used as antigen presenting cells (APC). When enriched dendritic cells (DC) were used as APC, CD4+ T cells from GM-CSF −/− mice proliferated as well as those from GM-CSF +/+ mice and produced high amounts of IFN-γ and IL-4. To analyze the rescue effect of DC on CD4+ T cells, supernatants from (i) CD4+ T cells cultured with KLH-pulsed DC or (ii) DC cultured with recombinant GM-CSF were transferred to cultures of CD4+ T cells and KLH-pulsed spleen cells from GM-CSF −/− mice. Supernatants from both DC sources contained a factor or factors that restored proliferative responses and IFN-γ production of CD4+ T cells from GM-CSF −/− mice.
Granulocyte/macrophage colony-stimulating factor (GM-CSF) has received much attention since the cloning of mouse and human GM-CSF and the greater availability of the recombinant product. Studies with recombinant GM-CSF (rGM-CSF) substantiated initial observations showing the effect of GM-CSF on the proliferation and maturation of myeloid progenitor cells in vitro and the increase in granulocytes and monocytes in peripheral blood after GM-CSF injection (1, 2). GM-CSF has also been found to be critical for the in vitro differentiation and proliferation of dendritic cells (DC) from hematopoietic precursor cells (3). Although the hematopoietic activities of GM-CSF have received the most attention, there is growing interest in the immunological effects of this cytokine, particularly its use as an immunological adjuvant (4) and its ability to augment immune responses to tumor antigens. In a study of mouse B16 melanoma, Dranoff et al. (5) compared the relative immunogenicity of B16 cells transduced with retroviral vectors coding for various cytokines. In a comparison of seven different cytokines, immunization with GM-CSF producing B16 cells showed the greatest protection against subsequent challenge with parental B16 cells; this immunity was mediated by CD4+ and CD8+ T cells (5). Tao and Levy (6) reported that immunization with an idiotype/GM-CSF fusion protein produced higher levels of anti-idiotype antibodies and greater protection against an idiotype positive B cell lymphoma than immunization with idiotype alone, idiotype mixed with GM-CSF, or idiotype with adjuvant. Jäger et al. (7) have recently shown that rGM-CSF can enhance the generation of cytotoxic T lymphocytes (CTL) and development of hypersensitivity after immunization with peptides derived from melanoma differentiation antigens.
The availability of GM-CSF deficient mice now makes it possible to define more precisely the role of this cytokine in immune responses. In the present study, we examined T and B cell functions in mice lacking GM-CSF.
MATERIALS AND METHODS
Mice.
GM-CSF-deficient mice (GM-CSF −/− mice) on a C57BL/6 × 129 background were generated at the Melbourne Branch of Ludwig Institute for Cancer Research (8), and breeding stocks were transferred to the New York Branch. (C57BL/6 × 129)F2 mice were used as controls for GM-CSF −/− mice. BALB/c mice were obtained from the breeding facility at Memorial Sloan–Kettering Cancer Center.
mAbs.
Anti-L3T4 (CD4) and anti-Lyt2.2 (CD8) mAbs were kindly provided by F. Fitch (University of Chicago) and U. Hämmerling (Memorial Sloan–Kettering Cancer Center), respectively. Other mAbs used in this study were purchased from PharMingen.
Tumor Cell Lines.
EL4 is a chemically induced leukemia cell line of C57BL origin. RL♂1 is a BALB/c radiation-induced leukemia.
Peptide.
The ovalbumin (OVA) peptide spanning residues 257–264 (SIINFEKL) was synthesized and purified by Bio-Synthesis (Lewisville, TX) (9).
OVA Peptide Immunization.
OVA peptide (5 μg) in TiterMax (CytRx, Norcross, GA) was injected in the hind footpads. In mice receiving rGM-CSF, 5 ng rGM-CSF (PharMingen) was injected in the hind footpads with peptide followed by 10 ng rGM-CSF injected i.p. for 5 days.
Keyhole Limpet Hemocyanin (KLH) Immunization.
Mice were immunized with 100 μg KLH (Pierce) in complete Freund’s adjuvant (CFA) (Sigma) in the hind footpads. In mice receiving rGM-CSF, 40 ng rGM-CSF was injected in the hind footpads with KLH followed by 10 ng rGM-CSF injected i.p. for 5 days a week until mice were killed.
Generation of CTL Specific to OVA Peptide.
Details have been described elsewhere (9).
Mixed Lymphocyte Reaction.
For proliferation assays, 3 × 105 responding spleen cells (H-2b background) were cultured with 2 × 105 mitomycin C (Sigma)-treated BALB/c mice spleen cells for 4 days at 37°C in a 5% CO2 atmosphere. Proliferation was determined by incorporation of [methyl-3H]thymidine. For generation of H-2d specific-CTL, 3 × 107 responding spleen cells were cultured with 2 × 107 mitomycin C-treated BALB/c mice spleen cells for 5 days at 37°C in a 5% CO2 atmosphere. Cytotoxicity was assessed in 51Cr-release assays using BALB/c RL♂1 as target cells.
T Cell Responses to KLH.
Mice were killed 7–10 days after immunization and the popliteal lymphnodes were removed. To obtain purified CD4+ T cells, lymphnode cells were treated with anti-Lyt 2.2 mAb and rabbit serum as complement and then passed through a nylon wool column (>90% cells were Thy-1+CD4+CD8−). Using spleen cells or purified B cells as antigen-presenting cells (APC), the cells were pulsed with 100 or 10 μg/ml KLH and 50 μg/ml mitomycin C for 1 h and washed, and 1 × 106 APC were then cultured with 1 × 105 CD4+ T cells at 37°C in a 5% CO2 atmosphere for 3 days. Using DC as APC, the cells were pulsed with 10 μg/ml KLH overnight and treated with 50 μg/ml mitomycin C for 1 h before washing. DC (1 × 105) were then cultured with 1 × 105 CD4+ T cells at 37°C in a 5% CO2 atmosphere for 3 days. To analyze T cell responses to nonspecific stimulants, 1 × 105 CD4+ T cells were cultured with immobilized anti-mouse CD3 mAb or concanavalin A (Con A) (Sigma) for 3 days. For experiments involving supernatants, 100 μl culture supernatant from cocultures of CD4+ T cells and KLH-pulsed DC (mitomycin C untreated) for 2 days or from cultures of 5 × 105 DC stimulated with 100 ng/ml rGM-CSF in 96-well plates for 3 days were transferred to cultures of CD4+ T cells and KLH-pulsed spleen cells from GM-CSF −/− mice at a final volume of 200 μl.
Cytokine Assays.
Culture supernatants were assayed by ELISA for interferon-γ (IFN-γ) and interleukin 4 (IL-4) using reagents from PharMingen, and for IL-2 using the DuoSet reagent from Genzyme.
ELISA for Detection of Antibodies Against KLH.
KLH was dissolved in 0.1 M sodium bicarbonate at pH 8.3 at a concentration of 1 μg/ml. A solution of 100 μl KLH was added to each well of 96-well plates and incubated at 4°C overnight. After extensive washing with PBS, 100 μl 2% BSA in PBS was added and incubated overnight. Serially diluted serum samples were then added and incubated at room temperature for 2 h. A total of 100 μl of alkaline phosphatase-labeled mouse IgM-specific or mouse IgG subclass-specific antibodies (Abs) (diluted at 1:5,000 with PBS) was added after washing and incubated at room temperature for 1 h. Substrate solution (100 μl) (JBL Scientific, San Luis Obispo, CA) was added after the final wash and incubated at 37°C for 20 min, and plates were then read by CytoFluor (Millipore) with 450 nm wavelength at excitation and 580 nm wavelength at emission. Specificity of Abs was confirmed using hen egg lysozyme (Sigma) as a negative control.
Purification of B Cells and DC.
For B cell purification, spleen cells were centrifuged over 55% percoll (Sigma) after depletion of T cells using anti-L3T4 mAb, anti-Lyt 2.2 mAb, and rabbit serum as complement. Mac-1+ and CD11c+ cells at the interface between media and 55% percoll were eliminated using biotinylated anti-Mac-1 and CD11c mAb and avidin-coated magnetic beads. The remaining cells were used as enriched B cells (>80% B220+, <1% Mac-1+, <0.6% CD11c+) (10). For DC purification, collagenase-treated spleen cells were centrifuged over 55% percoll after T cell depletion using anti-Thy1 mAb and complement. B-220+ cells at the interface were eliminated using biotinylated anti-B220 mAb and avidin-coated magnetic beads. The remaining cells were cultured in 60 mm Petri dishes at 37°C in a 5% CO2 atmosphere overnight, and nonadherent cells were then used as enriched DC (>45% CD11c+ IAb++ CD16/32−, <2.0% Mac-1++ CD16/32+) (10). For DC cultures with rGM-CSF, mice were injected with 100 μg lipopolysaccharide (LPS) (Sigma) and spleens were removed 3 h later. CD11c+ cells were isolated from spleen cells using biotinylated anti-CD11c mAb and avidin-coated magnetic beads.
RESULTS
GM-CSF −/− Mice Respond Normally to Alloantigens.
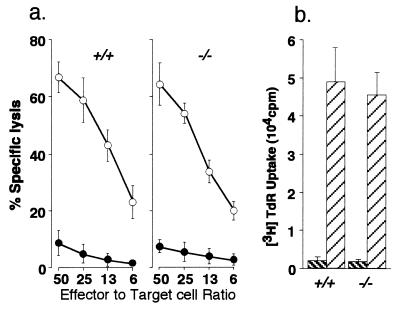
As a first step, we analyzed the generation of allo-specific CTL from naive GM-CSF −/− and GM-CSF +/+ spleen cells after coculture with BALB/c spleen cells. As shown in Fig. 1a, CTL from GM-CSF −/− mice lysed BALB/c target cells as effectively as those from GM-CSF +/+ mice. Fig. 1b shows the proliferative responses of spleen cells from GM-CSF −/− and GM-CSF +/+ mice against BALB/c stimulatory cells. Cells from GM-CSF −/− mice proliferated as well as those from GM-CSF +/+ mice. The proliferative response of splenic T cells as well as isolated CD4+ T cells from GM-CSF −/− and GM-CSF +/+ mice to anti-mouse CD3 mAb, Con A, and IL-2 was also comparable.
Figure 1.
CTL and proliferative responses of naive GM-CSF +/+ and GM-CSF −/− spleen cells against BALB/c spleen cells. Spleen cells from GM-CSF +/+ (+/+) or GM-CSF −/− (−/−) mice were stimulated in vitro with mitomycin C-treated BALB/c spleen cells. (a) Cytotoxicity was assessed with 51Cr-labeled BALB/c RL♂1 (○) and C57BL EL4 (•) target cells. (b) Spleen cells from GM-CSF +/+ or GM-CSF −/− mice were stimulated in vitro with mitomycin C-treated autologous (▧) or BALB/c (▨) spleen cells. Proliferation was determined by incorporation of [methyl-3H]thymidine.
Failure of CTL Response to OVA Peptide.
We next evaluated CD8+ T cell responses of GM-CSF −/− mice against an exogenous peptide. OVA peptide spanning residues 257–264 (SIINFEKL) is recognized by CTL in a Kb-restricted manner, and CTL can be generated without CD4+ T cell help (9). GM-CSF −/− and GM-CSF +/+ mice were immunized with OVA peptide in adjuvant. Seven days after immunization, mice were killed and spleen cells were cultured for 5 days with mitomycin C-treated autologous spleen cells pulsed with OVA peptide. Effector cells were assayed for cytotoxicity in 51Cr-release assay using EL4 cells (H-2b) pulsed with OVA peptide. As shown in Fig. 2a, highly reactive CTL specific to OVA peptide were elicited from GM-CSF +/+ mice but not from GM-CSF −/− mice (Fig. 2b). When GM-CSF−/− mice were injected with rGM-CSF during the immunization period, reactive CTL against OVA peptide were generated (Fig. 2c).
Figure 2.
Generation of CTL against OVA peptide. Spleen cells of GM-CSF +/+ (a) or GM-CSF −/− mice (b and c) immunized with OVA peptide were stimulated with autologous spleen cells pulsed with OVA peptide and treated with mitomycin C. Cytotoxicity was assessed with 51Cr-labeled C57BL EL4 target cells pulsed with OVA peptide (○) or with no peptide (•). (c) The hind footpads of GM-CSF −/− mice were injected with 5 ng rGM-CSF and OVA peptide, followed by 10 ng/day rGM-CSF injected i.p. for 5 days, resulting in the generation of OVA-specific CTL .
Delayed Ab Production Against KLH.
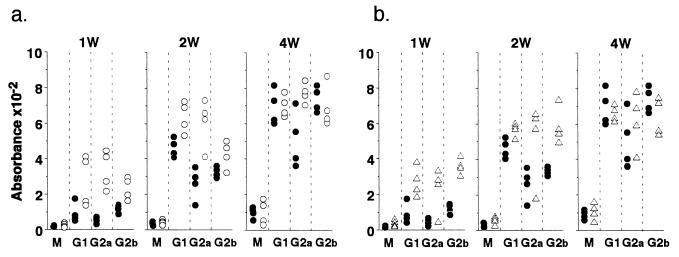
We next asked whether GM-CSF −/− mice respond normally to exogenous soluble proteins. For this purpose, GM-CSF −/− and GM-CSF +/+ mice were immunized with 100 μg KLH in CFA and bled 1 week, 2 weeks, and 4 weeks after immunization. IgM, IgG1, IgG2a, and IgG2b titers against KLH were quantitated by ELISA. As shown in Fig. 3a, GM-CSF −/− mice produced lower levels of IgG, particularly IgG2a, during the first 2 weeks following immunization. However by 4 weeks, only IgG2a levels remained suppressed in GM-CSF −/− mice. IgM production was not compromised in GM-CSF −/− mice throughout the observation period. Injection of rGM-CSF in vivo restored IgG production in GM-CSF −/− mice (Fig. 3b). These observations were confirmed using lower levels of immunization (10 μg and 1 μg KLH).
Figure 3.
Ab responses to KLH. (a) The hind footpads of GM-CSF −/− (•) and GM-CSF +/+ (○) mice were injected with 100 μg KLH in CFA. (b) The hind footpads of GM-CSF −/− mice were injected with 100 μg KLH with 40 ng rGM-CSF in CFA followed by 10 ng/day rGM-CSF injected i.p. for 5 days a week until mice were killed: GM-CSF −/− (•) and GM-CSF −/− with rGM-CSF (▵). Each group had four mice. Individual mice were bled at 1, 2, and 4 weeks after immunization and sera were titered for anti-KLH antibody at doubling dilutions of 1:500 to 1:16,000 using isotype-specific ELISA. The results of serum samples diluted 1:4,000 are plotted in this figure. Similar differences were seen with the other serum dilutions. Specificity of antibodies were confirmed using hen egg lysozyme as a negative control.
Impaired Proliferative Responses of CD4+ T Cells to KLH.
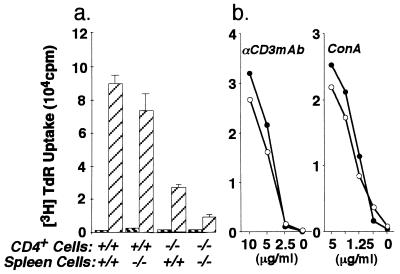
Fig. 4 shows the proliferative responses of CD4+ T cells from GM-CSF −/− and GM-CSF +/+ mice immunized with KLH. With spleen cells as APC, the proliferative response of CD4+ T cells from GM-CSF −/− mice was clearly impaired (Fig. 4a). To distinguish whether this defect is at the level of CD4+ T cells or APC, CD4+ T cells from GM-CSF +/+ mice and KLH-pulsed spleen cells from GM-CSF −/− mice or CD4+ T cells from GM-CSF −/− mice and KLH-pulsed spleen cells from GM-CSF +/+ mice were cocultured. As shown in Fig. 4a, CD4+ T cells from GM-CSF +/+ mice cultured with APC from GM-CSF −/− mice proliferated as well as those cultured with APC from GM-CSF +/+, whereas CD4+ T cells from GM-CSF −/− cultured with APC from GM-CSF +/+ mice showed impaired proliferation, indicating that CD4+ T cells rather than APC are responsible for the low proliferative response against KLH in GM-CSF −/− mice. This defect is antigen specific, because CD4+ T cells from immunized GM-CSF −/− mice showed a normal response to anti-mouse CD3 mAb or Con A (Fig. 4b). With regard to cytokine production, the pattern of cytokines produced by CD4+ T cells from immunized GM-CSF −/− and GM-CSF +/+ mice could be distinguished. As shown in Table 1, CD4+ T cells from GM-CSF +/+ mice cocultured with APC showed high production of IFN-γ and IL-4 and low production of IL-2. In contrast, CD4+ T cells from GM-CSF −/− mice produced low IFN-γ and IL-4 and high IL-2. Proliferative responses against KLH and IFN-γ and IL-4 production were partially restored by injection of rGM-CSF (Table 1).
Figure 4.
CD4+ T cell proliferative responses to KLH, immobilized anti-CD3 mAb, and Con A following immunization with KLH in CFA. (a) Purified CD4+ T cells from draining lymphnodes of immunized GM-CSF −/− or GM-CSF +/+ mice were stimulated with spleen cells pulsed with KLH (▨) or without KLH (▧) as indicated. Spleen cells were pulsed with 100 μg/ml KLH for 1 h. (b) Purified CD4+ T cells from immunized GM-CSF −/− (•) or GM-CSF +/+ (○) mice were stimulated with immobilized anti-CD3 mAb or Con A in vitro. Proliferation was determined by incorporation of [methyl-3H]thymidine.
Table 1.
Proliferation and cytokine production of CD4+ T cells from GM-CSF −/− and +/+ mice immunized with KLH
| CD4+ cell | APC* | Pulsed KLH, μg/ml | [3H]Thymidine uptake,† cpm ± SD | IFN-γ, units/ml | IL-2, pg/ml | IL-4, units/ml |
|---|---|---|---|---|---|---|
| Exp. 1 | ||||||
| +/+ | Spleen cells | 100 | 68,437 ± 12,395 | 46.9 | 65.3 | 218.3 |
| −/− | Spleen cells | 100 | 10,386 ± 3,284 | 3.4 | 139.3 | 30.5 |
| Exp. 2 | ||||||
| +/+ | B cells | 10 | 62,285 ± 15,689 | 85.6 | 20.5 | 59.2 |
| −/− | B cells | 10 | 8,477 ± 2,475 | <2.0 | 70.3 | 6.6 |
| −/−‡ | B cells‡ | 10 | 39,884 ± 6,008 | 30.1 | 22.1 | 24.3 |
Results shown here come from the analysis of individual mice. Comparable results have been obtained in repeated experiments.
Autologous spleen cells or B cells were used as APC.
Mean ± SD of triplicate determinations using CD4+ T cells and APC from the same mouse.
CD4+ T cells and B cells were from GM-CSF −/− mice supplemented with rGM-CSF in vivo.
Normalization of CD4+ T Cell Responses by DC or by Transfer of Supernatants from DC Cultures.
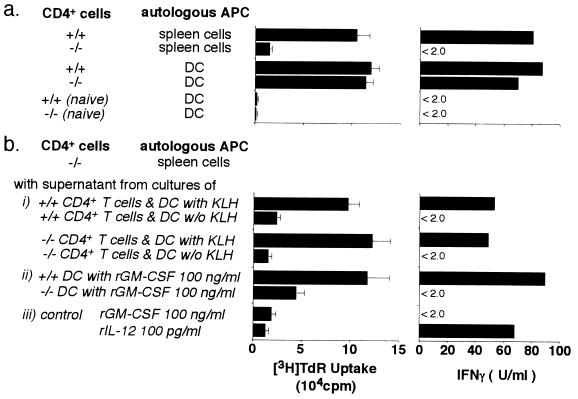
We next asked whether DC, which are known to be highly effective APC, could restore the proliferative response and IFN-γ production of CD4+ T cells from GM-CSF −/− mice. As shown in Fig. 5a, CD4+ T cells from GM-CSF −/− mice showed vigorous proliferation against KLH and produced high levels of IFN-γ when DC from GM-CSF +/+ or GM-CSF −/− mice were used as APC. To analyze this rescue effect of DC, supernatants were harvested from cultures of CD4+ T cells and KLH-pulsed DC from KLH immunized GM-CSF −/− or GM-CSF +/+ mice. These supernatants were transferred to cultures of CD4+ T cells and KLH-pulsed spleen cells from KLH-immunized GM-CSF −/− mice. Fig. 5b shows that the proliferative response and IFN-γ production of CD4+ T cells from GM-CSF −/− mice were completely restored by supernatants from GM-CSF −/− or GM-CSF +/+ DC cultures. For further analysis, CD11c+ cells were isolated from GM-CSF +/+ mice injected with 100 μg LPS and cultured with 100 ng/ml rGM-CSF for 3 days. The supernatants from these isolated CD11c+ cells were added to cultures of CD4+ T cells and KLH-pulsed spleen cells from KLH-immunized GM-CSF −/− mice. Under these conditions, CD4+ T cells proliferated vigorously and produced high amounts of IFN-γ, suggesting that these activated DC produce a factor or factors that can rescue T cell responses. Supernatants from DC isolated from GM-CSF +/+ mice not injected with LPS did not restore proliferative activity or cytokine production, nor could these activities be restored by supernatants of DC from LPS-injected GM-CSF −/− mice cultured with rGM-CSF. GM-CSF and IL-12 could not duplicate the effect of DC supernatants on the proliferative response and the cytokine production of GM-CSF −/− CD4+ T cells (Fig. 5b).
Figure 5.
DC and culture supernatants of DC stimulate the proliferative responses and IFN-γ production by CD4+ T cells from KLH-immunized GM-CSF −/− mice. (a) CD4+ T cells from KLH-immunized or naive mice were stimulated with autologous spleen cells or DC pulsed with KLH as indicated. (b) To analyze the effect of supernatants from DC, 100 μl supernatants were added to cultures of CD4+ T cells and KLH-pulsed spleen cells from immunized GM-CSF −/− mice at a final volume of 200 μl. Two sources of DC supernatants were used; (i) cocultures of immunized GM-CSF +/+ or GM-CSF −/− CD4+ T cells and KLH-pulsed autologous DC (mitomycin C-untreated) for 2 days, and (ii) cultures of DC from LPS-treated GM-CSF +/+ or GM-CSF −/− mice with 100 ng/ml rGM-CSF for 3 days. (iii) For control purposes, the effects of rGM-CSF or IL-12 added to cultures of CD4+ T cells and KLH-pulsed spleen cells from immunized GM-CSF −/− mice were also tested. Proliferation was determined by incorporation of [methyl-3H]thymidine and levels of IFN-γ in the culture supernatants were measured by ELISA.
DISCUSSION
In the present study, we investigated T cell-dependent immunological functions in GM-CSF −/− mice. T cells from naive GM-CSF −/− mice responded normally to allo-antigens and proliferated as vigorously as T cells from GM-CSF +/+ mice to immobilized anti-CD3 mAb, Con A, and IL-2, indicating that signals through the T cell receptor (TCR) are transmitted normally and that the intrinsic proliferative potential of CD4+ T cells is intact in GM-CSF −/− mice. However, the proliferative response of CD4+ T cells from immunized GM-CSF −/− mice to specific antigens was clearly impaired, indicating that T cell functions are compromised in an antigen-specific manner. This deficiency is not mediated by a direct effect of GM-CSF on T cells, because T cells lack receptors for GM-CSF (11) and because the defective proliferative response of T cells from immunized mice could not be reversed by in vitro supplementation with rGM-CSF. The fact that in vivo administration of rGM-CSF is corrective, however, indicates that the effect of GM-CSF on T cells is indirect.
Several steps are required for the generation of a specific T cell response, starting with antigen processing and presentation of antigenic peptides by the major histocompatibility complex on APC, followed by T cell priming from signals transmitted through the TCR and through costimulatory molecules (such as CD28 and CD40 ligand), resulting in the clonal expansion of T cells (12, 13). CD4+ T cells from KLH-immunized GM-CSF −/− mice are demonstratively primed because high titer IgG can be formed (although delayed), and the GM-CSF −/− CD4+ T cells cultured with KLH-pulsed DC proliferate as well as with those from GM-CSF +/+ mice. These experiments also showed that DC from GM-CSF −/− mice are fully competent to function as APC. The proliferative defect in CD4+ T cells of immunized mice is clearly evident when other sources of APC are tested. CD4+ T cells from GM-CSF −/− mice cocultured with KLH pulsed spleen cells or B cells proliferate poorly and produce low levels of IFN-γ and IL-4. Supernatants from these GM-CSF −/− CD4+ T cells cultured with KLH-pulsed spleen cells or B cells show high levels of IL-2, most likely due to low IL-2 consumption, in contrast to low IL-2 levels (presumably higher IL-2 consumption) in comparable cultures of GM-CSF +/+ CD4+ T cells. Thus, antigen-specific steps involving interactions of APC and T cells as well as signals transmitted through TCR and costimulatory molecules that lead to IL-2 production by T cells was clearly intact in GM-CSF −/− mice. This suggests that the low proliferative response of CD4+ T cells from immunized GM-CSF −/− mice is due to a low responsiveness of T cells to IL-2, and that the slower kinetics of IgG production in GM-CSF −/− mice results from insufficient CD4+ T cell help to B cells as a consequence of delayed clonal expansion of T cells in vivo. In the cognate interaction between T cells and APC, signals are generated that have a positive (e.g., CD28-B7) or negative (CTLA4-B7) influence on the resulting immune response (13, 14). Because it is known that GM-CSF is secreted by T cells, GM-CSF is likely to play a role in this cognate interaction. As DC can correct the proliferative defect of GM-CSF −/− CD4+ T cells, we postulated that GM-CSF either directly or indirectly induces DC to produce a factor or factors that enhances responsiveness to IL-2. Evidence in support of this idea comes from our finding that supernatants from DC cultures contain a factor or factors that restores the defect of GM-CSF −/− CD4+ T cells. The potent activity of DC as APC has been attributed to their high expression of MHC class II and critical costimulatory molecules, as well as their migratory capacity (15). Efforts have also been made to identify factors produced by epidermal Langerhans cells and DC (16, 17) that might be involved in their APC activity. Macatonia et al. (18) reported that IL-12 was produced by mouse CD11c+ DC, favoring development of T cells along the Th1 pathway. It has also been reported that human CD1a+ DC produce a soluble factor that induces the growth and differentiation of CD40-activated B cells (19). Another factor, the recently described DC-derived chemokine DC-CK-1, shows chemotactic activity for naive T cells, indicating that this chemokine may play a significant role in the initiation of immune responses (20). These findings point to the importance of soluble factors, in addition to signals transmitted through membrane-bound molecules, in the interaction between DC and T or B cells.
Although the factor presented in this paper is not characterized, the restoration of responsiveness of GM-CSF −/− CD4+ T cells can be used to guide purification and cloning. This DC-derived factor (which we designate DC-DF) is produced by DC activated by GM-CSF, or by signals transmitted through interaction between DC and CD4+ T cells that occur even in the absence of GM-CSF. Based on the fact that T cell function is impaired in an antigen-specific manner in GM-CSF −/− mice, it is reasonable to speculate that the absence of DC-DF induced by GM-CSF distorts the balance between the positive and negative signals exerted on T cells by antigen stimulation, resulting in the diminished responsiveness of T cells to IL-2. Thus, DC-DF enhances either the positive signals or neutralizes the inhibitory signals that control the proliferative drive of antigen-specific T cells in vivo and in vitro. DC-DF is not IL-12, because IL-12 had no effect on the proliferative response of KLH-specific CD4+ T cells from GM-CSF −/− mice. Molecular characterization of DC-DF will be necessary to show whether it is a new factor or related to known factors.
Acknowledgments
We thank Drs. Antony W. Burgess and Jonathan Cebon for valuable comments.
ABBREVIATIONS
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- DC
dendritic cells
- APC
antigen-presenting cells
- CTL
cytotoxic T lymphocytes
- rGM-CSF
recombinant GM-CSF
- Con A
concanavalin A
- OVA
ovalbumin
- KLH
keyhole limpet hemocyanin
- CFA
complete Freund’s adjuvant
- TCR
T cell receptor
- LPS
lipopolysaccharide
- IFN-γ
interferon-γ
- IL
interleukin
References
- 1.Moore M A S. Annu Rev Immunol. 1991;9:159–191. doi: 10.1146/annurev.iy.09.040191.001111. [DOI] [PubMed] [Google Scholar]
- 2.Lieschke G J, Burgess A W. N Engl J Med. 1992;327:28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- 3.Witmer-Pack M D, Olivier W, Valinsky J, Schuler G, Steinman R M. J Exp Med. 1987;166:1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disis M L, Bernhard H, Shiota F M, Hand S L, Gralow J R, Huseby E S, Gillis S, Cheever M A. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 5.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H I, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao M-H, Levy R. Nature (London) 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 7.Jäger E, Ringhoffer M, Dienes H P, Arand M, Karbach J, Jäger D, Ilsemann C, Hagedorn M, Oesch F, Knuth A. Int J Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Stanley E, Lieschke G J, Grail D, Metcalf D, Hodgson G, Gall J A M, Maher D W, Cebon J, Sinickas V, Dunn A R. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyall R, Vasovic L V, Molano A, Nikolic-Zugic J. Int Immunol. 1995;7:1205–1212. doi: 10.1093/intimm/7.8.1205. [DOI] [PubMed] [Google Scholar]
- 10.Guery J-C, Ria F, Adorini L. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park L S, Friend D, Gillis S, Urdal D L. J Biol Chem. 1986;261:4177–4183. [PubMed] [Google Scholar]
- 12.Grewal I S, Xu J, Flavell R A. Nature (London) 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone J A. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 14.Paul W E, Sedar R A. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 15.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 16.Heufler C, Topar G, Koch F, Trockenbacher B, Kampgen E, Romani N, Schuler G. J Exp Med. 1992;176:1221–1226. doi: 10.1084/jem.176.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrick J W, Morhenn V, Chiang Y L, Shi T. J Leukocyte Biol. 1989;45:429–433. doi: 10.1002/jlb.45.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Macatonia SE, Hosken N A, Litton M, Vieira P, Hsieh C-S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 19.Dubos B, Vanbervliet B, Fayette J, Massacrier C, Kooter C V, Briere F, Banchereau J, Caux C. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adema G J, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon K B, Figdor C G. Nature (London) 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]