Abstract
The Dld gene product, known as dihydrolipoamide dehydrogenase or the E3 component, catalyzes the oxidation of dihydrolipoyl moieties of four mitochondrial multienzyme complexes: pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, branched-chain α-ketoacid dehydrogenase, and the glycine cleavage system. Deficiency of E3 activity in humans results in various degrees of neurological dysfunction and organic acidosis caused by accumulation of branched-chain amino acids and lactic acid. In this study, we have introduced a null mutation into the murine Dld gene (Dldtm1mjp). The heterozygous animals are shown to have approximately half of wild-type activity levels for E3 and all affected multienzyme complexes but are phenotypically normal. In contrast, the Dld−/− class dies prenatally with apparent developmental delay at 7.5 days postcoitum followed by resorption by 9.5 days postcoitum. The Dld−/− embryos cease to develop at a time shortly after implantation into the uterine wall when most of the embryos have begun to gastrulate. This null phenotype provides in vivo evidence for the requirement of a mitochondrial oxidative pathway during the perigastrulation period. Furthermore, the early prenatal lethal condition of the complete deficiency state may explain the low incidence of detectable cases of E3 deficiency in humans.
Keywords: gene targeting, embryonic lethal mutation, embryonic metabolism
The product of Dld assembles into a dimeric flavin-requiring oxidoreductase that catalyzes the oxidation of dihydrolipoyl moieties of several noncovalently associated proteins (1). The Dld gene product performs this catalytic function for three mitochondrial multienzyme α-ketoacid dehydrogenase complexes: pyruvate dehydrogenase complex (PDC), α-ketoglutarate dehydrogenase complex (KDC), and branched-chain α-ketoacid dehydrogenase complex (BCKDC) (2). Because this reaction is the third step in catalysis of the α-ketoacid dehydrogenase complexes, this enzyme is often referred to as the E3 component. There also is evidence indicating that the Dld gene product participates in the glycine cleavage system (GCS) where it has been traditionally referred to as the L protein (3). The E3-dependent complexes catalyze key regulatory reactions in four different pathways of intermediary metabolism. PDC is essential for the oxidative metabolism of glucose and select amino acids because it links the glycolytic and tricarboxylic acid pathways through the decarboxylation of pyruvate to produce acetyl-CoA. KDC catalyzes one of the enzymatic steps of the tricarboxylic acid cycle, the central oxidative pathway for numerous metabolites, and a significant supplier of reducing equivalents to the electron transport chain. BCKDC catalyzes an irreversible step in the catabolism of three essential amino acids, leucine, isoleucine, and valine. GCS is involved in the synthesis and degradation of glycine as well as a source of one-carbon units for numerous metabolic pathways.
The majority of studies investigating early murine embryonic metabolism thus far have examined the metabolism in vitro of exogenous substrates, particularly glucose. In mouse embryos and other mammalian systems, it has been shown that the fate of consumed glucose varies during development. In the preimplantation period, murine embryos shift from oxidative metabolism to glycolytic metabolism of glucose (4). Embryos appear to continue to rely on glycolysis in the period immediately after implantation into the uterine wall with virtually all glucose, greater than 95%, taken up in culture is converted to lactate (5). The importance of glucose metabolism during the early postimplantation period in vivo has been established by the observation that embryos lacking the glycolytic enzyme glucose-6-phosphate isomerase die within several days after implantation, coinciding with the disappearance of maternally derived glucose-6-phosphate isomerase (6, 7). In the following days of postimplantation development, in vitro studies have demonstrated a gradual up-regulation of oxidative metabolism of glucose, although questions addressing the significance of these changes and their relevance to embryonic metabolism in vivo remain unanswered (5, 8). Direct evidence for the metabolism of amino acids during murine embryogenesis has not been established, but studies have shown that the addition of amino acids to culture medium may improve growth and/or viability of preimplantation embryos (9).
The generation of a complete deficiency state of E3 activity by gene targeting should provide a means to assess the importance of a combination of oxidative metabolic pathways during murine development. Although no mutations have been identified that affect E3 activity or any of the E3-dependent complexes in mice, there are several reports for each deficiency in humans. Patients with E3 deficiency suffer from various degrees of neurologic impairment that are often lethal within the first few years of life. To date, 10 cases of E3 deficiency, eight cases of KDC deficiency, and more than 100 cases of PDC deficiency have been reported in the literature (10–15). All of these cases have been diagnosed in the perinatal period or later, and no cases have been shown to completely lack enzymatic activity, suggesting the importance of PDC and KDC during prenatal development. In contrast, the limited biochemical/molecular characterizations of cases of BCKDC and GCS deficiencies suggest that complete deficiency states of these complexes are compatible with survival into the postnatal period (16, 17). This report describes the generation and phenotypic characterization of a null allele of the murine Dld gene, Dldtm1mjp.
MATERIALS AND METHODS
Construction of Targeting Vector.
A 13-kb mouse genomic clone was isolated from a 129SVJ genomic λ phage library (Stratagene) by using two fragments from the murine Dld gene as probes that were previously isolated from a DBA/2J genomic library (18). A 7-kb XbaI subclone representing the 3′ half of the genomic λ clone was subcloned into pUC19 vector (Life Technologies) to serve as the region of homology in the targeting vector (Fig. 1A). The Dld gene was disrupted at an EcoRI site located at codon 301 within exon 10 by inserting a β-actin promoter-driven neomycin phosphotransferase gene (neo) lacking a polyadenylylation signal (19). The cloning site is located upstream of key residues that have been shown in studies of purified human E3 protein to be essential for catalytic function (20). To select against random insertions of the construct, a diphtheria toxin A-fragment cassette (19) was inserted into the flanking region of homology. The construct was linearized by using a NotI site within the polylinker.
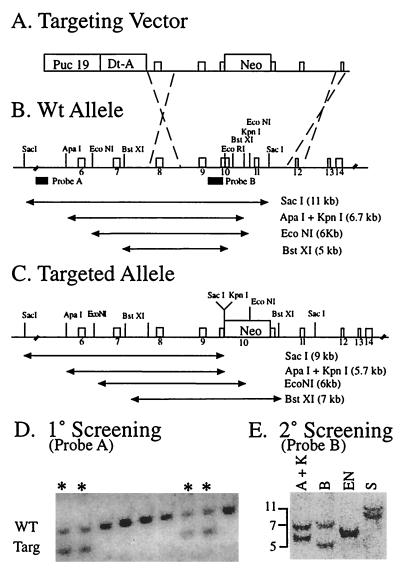
Figure 1.
Targeting of the Dld gene. (A) Linearized targeting vector is composed of the pUC19 vector, the diptheria toxin fragment A (Dt-A) negative marker, 7 kb of homology, and a neo cassette (neo). (B) The wild-type Dld allele is represented by numbered boxes indicating exons with restriction enzyme sites listed above. The two probes used for distinguishing the wild type from the targeted alleles are designated by the solid boxes. The sizes of the various restriction fragments and the enzymes used are shown below the restriction map. (C) The targeted allele after a recombination event is shown with the integrated neo cassette and the altered restriction map. (D) ES cell colonies after electroporation and selection were initially screened by using a SacI digest and probe A, which identified targeted alleles by a 2-kb reduction in size (lanes with asterisks). (E) The clones that appeared to be heterozygous by initial screen were subsequently analyzed by additional digests by using the internal probe B with lane designations as follows: A+K, ApaI+KpnI; B, BstXI; EN, EcoNI; S, SacI.
Generation and Analysis of Dld+/− Animals.
The E14.1 embryonic stem (ES) cell line (21) was cultured under routine conditions similar to those described by Ramirez-Solis et al. (22). ES cells were electroporated with linearized targeting vector, selected with G418, and screened by Southern blot analysis to identify recombinant clones (Fig. 1). Four independent Dld+/− ES cell lines were injected into C57BL/6 recipient blastocysts and transferred to the uteri of CH3B16 pseudopregnant females. The chimeric animals were bred with Black Swiss females to produce F1 progeny that were genotyped by Southern blot analysis or PCR using a trio of primers that simultaneously amplify regions of the wild-type and targeted alleles (Fig. 2C): 5′ common primer C1 (5′-GGTGGAATTGGAATTGACAT-3′), wild-type allele primer W1 (5′-TTATTGACTGGAATTCTACCTTTGGGATCT-3′), and targeted allele primer T2 (5′-ACCCCCTCTCCCCTCCTTTTG-3′).
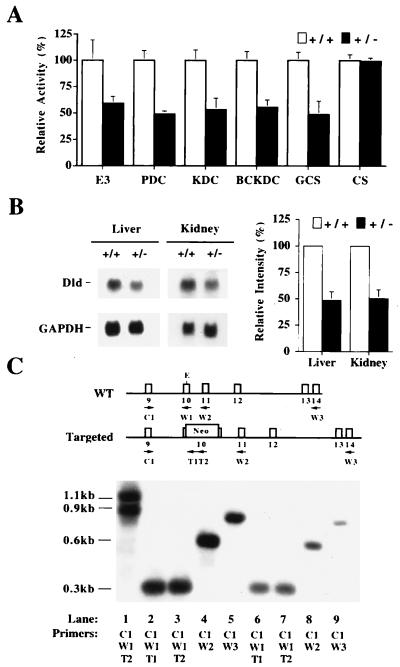
Figure 2.
Analysis of the effect of the Dldtm1mjp. (A) Liver homogenates from Dld+/+ and Dld+/− animals were assayed for E3 activity, each of the E3-dependent complexes and citrate synthase (CS) (n = 4 except for GCS where n = 3). The graph indicates the percentage of activity relative to wild-type levels: E3, 21.0 ± 3.0; PDC, 3.1 ± 0.3; KDC, 5.1 ± 0.4; BCKDC, 0.79 ± 0.05; GCS, 0.88 ± 0.06; CS, 0.17 ± 0.01 (expressed as mean ± SD in milliunits/mg of total protein, except for GCS where milliunits/mg of total mitochondrial protein was used). (B) Northern blot analysis of total RNA from liver and kidney samples of Dld+/+ and Dld+/− animals by using the full-length murine Dld cDNA as a probe. For control of loading, the blots were simultaneously probed with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe. A comparison of the normalized intensities with GAPDH is also presented. (C) RT-PCR analysis of total RNA from Dld+/+ and Dld+/− liver samples. The locations of the primer annealing sites are depicted above the blot according to the wild-type and targeted alleles: C1, common primer; W1, W2, and W3, wild-type specific primers for exons 10, 11, and 14, respectively; T1 and T2, mutant specific primers. The amplifications were size-fractionated by gel electrophoresis, blotted, and probed with the murine Dld cDNA. The primers used for each amplification are listed below the lanes. The templates used were as follows. Lanes: 1, Dld+/− genomic DNA; 2–5, Dld+/+ total RNA from liver; 6–9, Dld+/− total RNA from liver. The amplification products of genomic DNA for the wild-type and mutant alleles are 0.9 kb and 1.1 kb, respectively. The amplification products from primer pairs C1/W1, C1/W2, and C1/W3 are 0.3, 0.6, and 0.85 kb, respectively, for the wild-type allele cDNA. For the targeted allele, the amplication products from primer pairs C1/T1 and C1/T2 are 0.4 and 0.5 kb, respectively. E, EcoRI.
The Dld+/− F1 progeny from the chimera × Black Swiss matings were examined for any obvious phenotypic effect of carrying a mutant Dld allele by comparing behavior, gross anatomy, fertility, and growth curves with Dld+/+ littermates. Liver and kidney samples from the Dld+/+ and Dld+/− littermates were assayed for E3 activity as described by Patel et al. (23). The effects of the mutation on the E3-dependent multienzyme complexes were examined by assaying liver homogenates of Dld+/+ and Dld+/− littermates for each of the four complexes. Citrate synthase activity was also measured to assess mitochondrial function (24–28). To determine the effect of the disruption mutation on gene expression, Dld+/+ and Dld+/− animals were subjected to Northern blot analyses using the murine Dld cDNA as a probe and glyceraldehyde-3-phosphate dehydrogenase cDNA as a loading control (18). Lower levels of Dldtm1mjp transcript were assessed by reverse transcription-coupled PCRs (RT-PCR) using the set of primers previously described or with an alternative targeted allele primer T1 (5′-CCTCCGCCCTTGTGGACACT-3′). Any abnornally spliced products that removed the neo sequence were detected by primer pairs that amplify across the EcoRI site: W2 (5′-CTCCCAGCACTCTATCTGTT-3′) or W3 (5′-CTGCAAATTCTTGGAACTGG-3′) (Fig. 2C).
Identification and Analysis of the Dld−/− Embryos.
To assess the homozygous mutant phenotype, Dld+/− F1 animals were intercrossed and F2 progeny were genotyped. The stage of prenatal death of Dld−/− embryos was determined by dissecting out embryos at various stages of development, photographing, and genotyping them by PCR. Larger embryos at 7.5 days postcoitum (dpc) or later were genotyped by a single round of amplification with a trio of primers: a common intron 9 primer (5′-CACTAAGCTCCATCTTCAGCCATGAG-3′), a wild-type allele intron 10 primer (5′-GGTCTGTTTTTATCTTTAGAGAGAGCCAAAAA-3′), and a mutant allele β-actin promoter primer (5′-CCTCCGCCCTTGTGGACACT-3′). The smaller preimplantation embryos were genotyped by nested PCR in which an additional set of primers that were external to the original set of primers were used for the first round of amplification: C1, T2 (see above), and wild-type allele primer (5′-CCTTTACAATATACCCGCCTCACCAT-3′). To assess the mutant phenotype in more detail, decidua at several time points around the established time of embryonic death were embedded in paraffin, serially sectioned, and stained with hematoxylin/eosin.
RESULTS
Disruption of the Dld Allele.
A replacement targeting vector was designed in which the neo gene was inserted into the coding region within exon 10 (Fig. 1A). After electroporation, approximately 30% of the G418-resistant clones had undergone the desired homologous recombination event, as indicated by Southern blot analysis (Fig. 1D). Additional digestion assays of 5′ and 3′ flanking restriction sites demonstrated that the targeting vector had been inserted by homologous recombination into an endogenous gene. The absence of additional bands detected with an internal probe demonstrated that none of the lines carried additional randomly inserted copies of the targeting vector (Fig. 1E).
Three of the four injected lines produced chimeras that showed germ-line transmission of the ES cell genome. In analyzing the ES cell-derived progeny, the presence of a 1:1 ratio of Dld+/+:Dld+/− demonstrated that the introduced mutation had no dominant effects on viability. The Dld+/− animals were also similar to Dld+/+ littermates in gross appearance, behavior, and fertility. Liver samples were collected from the Dld+/+ and Dld+/− animals and analyzed by enzymatic assays to determine the effect of the mutation in vivo. The heterozygotes have approximately 50% of wild-type E3 activity levels compared with wild-type littermates, indicating that the disruption results in a loss of function (Fig. 2A). This half-fold change in E3 activity produces roughly similar reductions in each of the E3-dependent complexes in liver samples (Fig. 2A). As a control, the two genotypes were shown to have similar activity levels of the mitochondrial marker enzyme citrate synthase. Northern blot analysis showed that the Dld+/− animals have approximately 50% of wild-type levels of the 2.4-kb Dld mRNA with no additional bands being detected in liver and kidney samples (Fig. 2B). If the mutant transcript was processed by using the endogenous splice sites, then it would be expected to produce a transcript of 4.5 kb. To detect lower levels of mutant transcript, liver total RNA samples from Dld+/+ and Dld+/− animals were subjected to RT-PCR analysis using primers that amplify both wild-type and mutant alleles. No amplification product was detected with two different mutant-allele-specific primer pairs. The possibility of altered splicing patterns that remove all or part of the neo sequence was ruled out by demonstrating that primers amplifying across the neo insertion site detect only the wild-type amplification product (Fig. 2C).
Phenotypic Characterization of Dld−/− Animals.
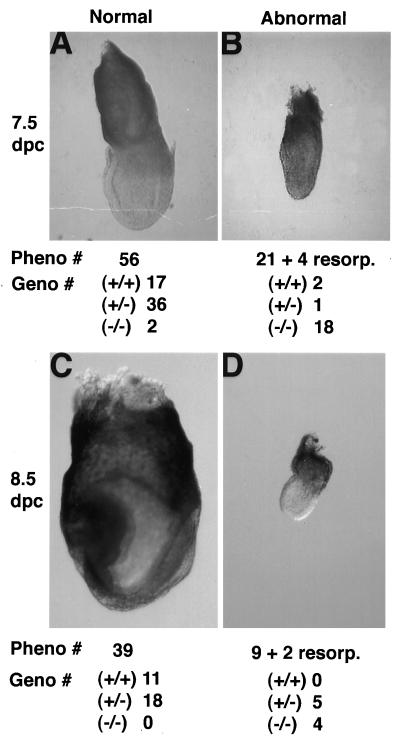
In the more than 200 progeny produced from intercrossing heterozygotes that originated from each of the three different clonal ES cell lines, the litters at birth were found to be approximately 25% smaller (7.6 ± 1.1 mice vs. 9.8 ± 1.6 mice) with the expected Mendelian ratio of Dld+/+ to Dld+/− (66:130) but with no Dld−/− class. The absence of Dld−/− mutants indicates that a recessive prenatal lethal allele has been created. To determine the time of death, embryos were collected at various stages of development and genotyped. At the blastocyst stage corresponding to 3.5 dpc, all embryos were similar in gross appearance with the expected Mendelian distribution of genotypic classes: 16:35:14 for Dld+/+/Dld+/−/Dld−/−. At 7.5 dpc, corresponding to several days after implantation, two phenotypic classes of embryos were noted upon removal from the decidua (Fig. 3 A and B). Approximately 75% of the embryos (56 of 81) appeared to be of expected size and morphology at pre- to early primitive streak stages. The remaining 25% of embryos were noticeably delayed in development because they were much smaller and resembled normal 6.5 dpc egg cylinders. The expected Mendelian ratio of genotypes at 7.5 dpc indicates that all Dld−/− embryos survive at least to this stage. As expected, the majority of phenotypically delayed embryos were genotyped as Dld−/−. The presence of two Dld−/− embryos that were phenotypically normal demonstrates some variation in expressivity among the mutant class. As would be expected, a small proportion of all embryos that contained a wild-type Dld allele were phenotypically abnormal due to some other cause of prenatal lethality.
Figure 3.
Identification of Dld−/− embryos. Embryos from a Dld+/− × Dld+/− mating were dissected from the decidua with the removal of Reichert’s membrane and ectoplacental cone tissue, photographed at ×15 magnification, and genotyped. The numbers below indicate the number of embryos identified with similar phenotypes and are then separated into genotypic classes.
About one quarter of the embryos at 8.5 dpc (Fig. 3 C and D) appeared to be similar in size to the mutant class at 7.5 dpc. Surprisingly, only approximately 50% of these embryos were genotyped as Dld−/− with the others being genotyped as Dld+/−. The presence of the wild-type allele amplification product in these samples was attributed to maternal contamination because these embryos were apparently being resorbed as noted by the large amounts of extravasated blood surrounding the embryos. The normal 8.5-dpc embryos, now at the head-fold stage of development, were genotyped as Dld+/+ or Dld+/− with a ratio of roughly 1:2, which further supports the notion that none of the Dld−/− class develop normally to 8.5 dpc. One day later at 9.5 dpc, 42 decidua were collected, of which 8 contained only resorption sites with insufficient embryonic material for genotypic analysis. These resorbed embryos were presumed to represent the Dld−/− class based on the 34 normally staged embryos being genotyped as Dld+/+ or Dld+/− with a ratio of 11:23.
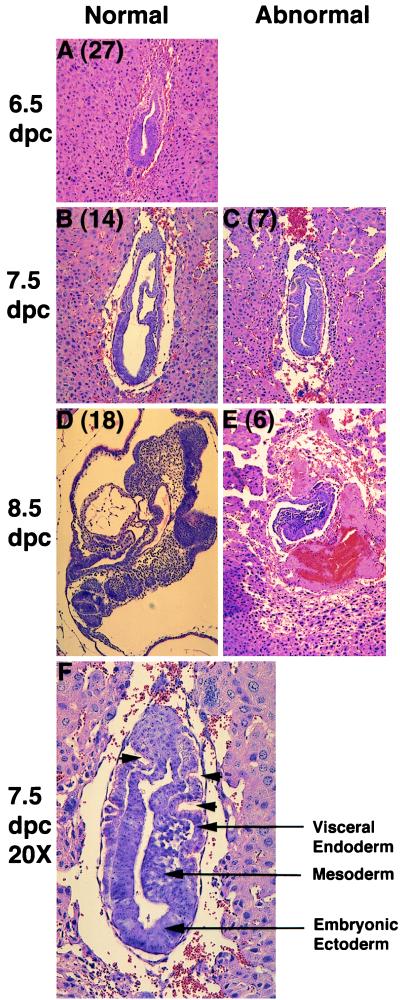
The mutant phenotype was examined in more detail though histologic analysis of decidua at 6.5 through 8.5 dpc with the results summarized in Fig. 4. Of the 28 embryos examined at day 6.5, all of the embryos with the exception of one grossly malformed embryo appear to be normal egg cylinders, suggesting that the Dld−/− class is not significantly delayed in growth or differentiation at this stage (Fig. 4A). By 7.5 dpc, 7 embryos out of the 21 examined were phenotypically abnormal (Fig. 4C). These abnormal embryos resembled 6.5-dpc egg cylinders with various degrees of disorganization. In 6 of the 7 abnormal embryos, there was evidence of mesoderm formation, which signals the onset of gastrulation (Fig. 4F). One unusual feature of these abnormal embryos was the presence of a thickened columnar layer of visceral endoderm. In normal 7.5-dpc embryos, the visceral endoderm assumes a unicellular flattened squamous morphology when in contact with the embryonic ectoderm. The presence of multiple folds in the extraembryonic region may be a result of either the initation of invaginations to form the three embryonic coela or continued proliferation of the visceral endoderm in a spatially confined area. The normal process of formation of the three coela can be appreciated in the normally developing 7.5-dpc embryo (Fig. 3B). One day later at 8.5 dpc, 75% of the embryos (18 of 24) were at the expected head-fold stage having initiated somitogenesis and organogenesis (Fig. 4D). The six abnormal embryos had not grown and were more disorganized relative to the abnormal embryos at 7.5 dpc. The hemorrhagic necrotic tissue surrounding these embryos indicates that these abnormal embryos were in the process of being resorbed by the maternal system (Fig. 4E).
Figure 4.
Histologic analysis of littermate embryos from Dld+/− × Dld+/− matings. The numbers in parentheses indicate the number of embryos with similar phenotypes (A–E, ×10). At 6.5 dpc, all embryos are considered normal because there were no obvious phenotypic differences (A). At subsequent days of development, the littermate embryos could be divided into two phenotypic classes (B–E). A higher magnification of an abnormal embryo at 7.5 dpc is included to allow for more detailed examination (F). Several important cell types are labeled and arrowheads indicate invaginations in the extraembryonic region.
DISCUSSION
The murine Dld gene was disrupted by the insertion of a neo gene into exon 10, which creates a loss of function mutation affecting gene expression predominantly at the mRNA level. The absence of detectable mRNA from the Dldtm1mjp allele in liver and kidney tissues as determined by Northern blot and RT-PCR analyses indicates that this mutation creates a null allele. The most likely explanation for the lack of detectable mutant transcript is mRNA instability. The phenomenon of mRNA instability caused by nonsense mutations has been documented in a number of different genes including β-globin (29) and triosephosphate isomerase (30). The recessive nature of this mutation is in accordance with data showing that human heterozygotes with approximately 50% of E3 activity levels are phenotypically normal (31). The half-fold reduction in E3 activity in Dld heterozygotes results in similar reductions in the activities of each of the E3-dependent complexes. The reduction in GCS activity is particularly noteworthy in light of past studies demostrating that rat liver mitochondria have immunologically distinct dihydrolipoamide dehydrogenase components for the GCS as compared with the α-ketoacid dehydrogenase complexes (32). The observed reduction of GCS activity in Dld+/− mice suggests that one gene codes for the dihydrolipoamide dehydrogenase of all four multienzyme complexes, which is also supported by studies of human heterozygotes (3).
In the homozygous state, the disrupted Dld allele appears to be incompatible with early postimplantation viability. At several days after implantation, Dld−/− embryos form egg cylinders but then become developmentally delayed. This finding was supported by histologic analyses of litters from heterozygote matings in which an obvious abnormal phenotype was first detected at 7.5 dpc. The aberrant 7.5-dpc embryos grossly resemble 6.5-dpc egg cylinders in size and proportion, indicating that virtually all cell types cease proliferation at the same time. One cell type, the visceral endoderm, appears to proliferate relatively longer and forms a folded thickened cell layer. The prolonged survival of this cell type may be related to its ability to uptake nutrients because it is believed to act as a specialized transport mechanism for macromolecules prior to development of the chorioallantoic placenta (33). Interestingly, recent in situ hybridization studies of early postimplantation mouse embryos have shown differences in the expression of various glucose transporter isoforms in the visceral endoderm compared with other embryonic cell types, which may reflect a differential ability to uptake this energy source (34). The survival of the Dld−/− embryos for almost 1 week postconception may be attributed to either the persistence of maternally encoded E3 protein or the lack of need for this enzyme before the perigastrulation period. There is a precedent for the persistence of a maternal protein into the postimplantation period based on the studies of glucose-6-phosphate isomerase (7). The persistance of maternal E3 protein is plausible considering both the long half-life of mitochondrial proteins that was shown to be 43 h for E3 protein in 3T3-L1 preadipocytes (35) and the large number of mitochondria per murine oocyte that has been estimated to be approximately 105 (36). Alternatively, metabolic studies in vitro provide evidence that the E3-dependent metabolic pathways may not be needed in the early postimplantation period based on the predominance of glycolysis (5, 8).
The time of developmental cessation in the Dld−/− embryos coincides with the onset of gastrulation, a period of rapid growth and differentiation in which the three germ layers are produced. Between 6.5 and 7.5 dpc, cell cycle times have been estimated to be as short as 5 h in embryonic tissue resulting in a 20-fold increase in cell number and a 14-fold increase in tissue volume (37). This large relative increase in biomass represents a significant energetic and biosynthetic demand on the embryo. Presently, it is not possible to point definitively to which affected pathways contribute to this phenotype. When the critical roles of PDC and KDC are considered, it is anticipated that deficiencies in these complexes would play a significant role in the Dld−/− phenotype. With the absence of these two enzymatic activities, the Dld−/− embryos would be unable to metabolize glucose oxidatively. The restriction to glycolysis would result in an ATP yield from glucose metabolism that would be only about 5% of that if glucose was metabolized oxidatively. Additionally, the loss of KDC activity would result in diminished concentrations of several of the tricarboxylic acid cycle intermediates that are required for the biosynthesis of essential molecules such as porphyrins and pyrimidines. The roles of BCKDC and GCS deficiencies in the Dld−/− phenotype are less clear. No data currently exists as to whether these complexes are expressed during early embryogenesis. One recent report has shown that a transcript of one of the GCS component genes can be detected by RT-PCR in porcine oocytes (38). The phenotypes of BCKDC and GCS deficiencies in humans result presumably from an accumulation of the unmetabolizable substrates to neurotoxic levels. It is unclear how relevant such effects would be during the perigastrulation period, a stage preceding neurogenesis, and when the small size of the embryo should permit molecules to readily diffuse into the maternal system.
Interestingly, it has been a long-held belief that early postimplantation development is essentially powered by anaerobic metabolism. The basis for this view began with histologic studies first performed by Krehbiel (39) that showed the primary decidual zone surrounding the implanted embryo to be devoid of patent vessels. Further support has been provided by the in vitro studies that have demonstrated virtually all glucose consumed by postimplantation embryos to be converted to lactate. Comparisons have been drawn between the rapidly developing embryonic tissue and other cell types such as transformed cells that rely on glycolysis presumably as a means of rapid ATP production. These studies have led to the hypothesis that oxidative metabolism does not play a significant role in postimplantation embryogenesis until after the formation of the chorioallantoic placenta when oxygen would become more readily available.
The Dld null phenotype provides direct evidence for the importance of oxidative metabolism during the early postimplantation period. When the 18:1 differentiation ATP yield in comparing aerobic to anaerobic glucose metabolism is considered, this result does not necessarily contradict the in vitro studies. Even the small changes in relative utilization of these two pathways recorded in vitro would translate to significant differences in total energy yield. The assumption of an anaerobic postimplantation milieu has been challenged recently by several studies, demonstrating the high permeability of the primary decidual zone (40). The permeable nature of this tissue may allow for adequate oxygenation merely by diffusion. Furthermore, Houghton et al. (41) have recently shown that early postimplantation embryos consume oxygen in vitro.
This report describes the characterization of a complete E3 deficiency in a mammalian system. Extrapolating to humans with the obvious reservations of such interspecies comparisons, the prenatal lethal Dld null phenotype may explain the low number of confirmed cases of E3 deficiency. These findings predict that only E3 deficiency states that preserve some residual activity would survive to postnatal life; a speculation supported by the fact that all of the reported cases of E3 deficiency, which were identified in the perinatal period or later, have detectable enzymatic activity. The availability of a Dld null allele in mice provides a valuable a system for investigating early embryonic metabolism and for introducing mutated transgenes to model the human postnatal disease states.
Acknowledgments
We thank Drs. Klaus Rajewsky and Clemencia Colmenares for the E14.1 ES cell line, Dr. John Duffy for performing the blastocyst injections, and Dr. Douglas Kerr for initial measurements of enzyme activities in liver samples. We acknowledge Drs. David Samols and Ron Conlon for critical reading of this manuscript. This work was supported by Metabolism Training Grant AM 07319 (M.T.J.) and U.S. Public Health Service Grant DK 42885 (M.S.P.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PDC, pyruvate dehydrogenase complex; KDC, α-ketoglutarate dehydrogenase complex; BCKDC, branched-chain α-ketoacid dehydrogenase complex; GCS, glycine cleavage system; dpc, days postcoitum; ES, embryonic stem; RT-PCR, reverse transcription–coupled PCR; neo, neomycin phosphotransferase.
References
- 1.Vettakkorumakankav N N, Patel M S. Ind J Biochem Biophys. 1996;33:168–176. [PubMed] [Google Scholar]
- 2.Reed L J. Acc Chem Res. 1974;7:40–46. [Google Scholar]
- 3.Yoshino M, Koga Y, Yamashita F. J Inherited Metab Dis. 1986;9:399–400. doi: 10.1007/BF01800493. [DOI] [PubMed] [Google Scholar]
- 4.Gardner D K, Leese H J. Human Reprod. 1986;1:25–27. doi: 10.1093/oxfordjournals.humrep.a136336. [DOI] [PubMed] [Google Scholar]
- 5.Clough J R, Whittingham D G. J Embryol Exp Morphol. 1983;74:133–142. [PubMed] [Google Scholar]
- 6.Kelly A, West J D. Dev Dyn. 1996;207:300–308. doi: 10.1002/(SICI)1097-0177(199611)207:3<300::AID-AJA7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.West J D, Leask R, Green J F. J Embryol Exp Morphol. 1986;97:225–227. [PubMed] [Google Scholar]
- 8.Wales R G, Martin K L, Leese H J. J Reprod Fertil. 1995;104:125–132. doi: 10.1530/jrf.0.1040125. [DOI] [PubMed] [Google Scholar]
- 9.Lane M, Gardner D K. J Reprod Fertil. 1997;109:153–164. doi: 10.1530/jrf.0.1090153. [DOI] [PubMed] [Google Scholar]
- 10.Robinson B H, Taylor J, Sherwood W G. Pediatr Res. 1977;11:1198–1202. doi: 10.1203/00006450-197712000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Liu T C, Kim H, Arizmendi C, Kitano A, Patel M S. Proc Natl Acad Sci USA. 1993;90:5186–5190. doi: 10.1073/pnas.90.11.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnefont J P, Chretien D, Rustin P, Robinson B, Vassault A, Aupetit J, Charpentier C, Rabier D, Saudubray J M, Munnich A. J Pediatr. 1992;121:255–258. doi: 10.1016/s0022-3476(05)81199-0. [DOI] [PubMed] [Google Scholar]
- 13.Guffon N, Lopez-Mediavilla C, Dumoulin R, Mousson B, Godinot C, Carrier H, Collombet J M, Divry P, Mathieu M, Guibaud P. J Inherited Metab Dis. 1993;16:821–830. doi: 10.1007/BF00714273. [DOI] [PubMed] [Google Scholar]
- 14.Kohlschutter A, Behbehani A, Langenbeck U, Albani M, Heidemann P, Hoffmann G, Kleineke J, Lehnert W, Wendel U. Eur J Pediatr. 1982;138:32–37. doi: 10.1007/BF00442325. [DOI] [PubMed] [Google Scholar]
- 15.Robinson B H. In: Lactic Acidemia (Disorders of Pyruvate Carboxylase, Pyruvate Dehydrogenase) Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1479–1499. [Google Scholar]
- 16.Chuang D T, Shih V E. In: Disorders of Branched Chain Amino Acid and Keto Acid Metabolism. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1239–1277. [Google Scholar]
- 17.Hamosh A, Johnston M V, Valle D. In: Nonketotic Hyperglycinemia. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1337–1348. [Google Scholar]
- 18.Johnson M, Yang H-S, Johanning G L, Patel M S. Genomics. 1997;41:320–326. doi: 10.1006/geno.1997.4670. [DOI] [PubMed] [Google Scholar]
- 19.Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Patel M S. J Biol Chem. 1992;267:5128–5132. [PubMed] [Google Scholar]
- 21.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. Nature (London) 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Solis R, Davis A C, Bradley A. Methods Enzymol. 1993;225:855–867. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- 23.Patel M S, Vettakkorumakankav N N, Liu T C. Methods Enzymol. 1995;252:186–195. doi: 10.1016/0076-6879(95)52022-8. [DOI] [PubMed] [Google Scholar]
- 24.Kerr D S, Berry S A, Lusk M M, Ho L L, Patel M S. Pediatr Res. 1988;24:95–100. doi: 10.1203/00006450-198807000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Chuang D T, Hu C W, Patel M S. Biochem J. 1983;214:177–181. doi: 10.1042/bj2140177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwim G W, Zhang B, Paxton R, Harris R A. Methods Enzymol. 1988;166:189–201. doi: 10.1016/s0076-6879(88)66025-3. [DOI] [PubMed] [Google Scholar]
- 27.Kochi H, Hayasaka K, Hiraga K, Kikuchi G. Arch Biochem Biophys. 1979;198:589–599. doi: 10.1016/0003-9861(79)90535-6. [DOI] [PubMed] [Google Scholar]
- 28.Srere P A. Methods Enzymol. 1969;7:3–5. [Google Scholar]
- 29.Ross J, Pizarro A. J Mol Biol. 1983;167:607–617. doi: 10.1016/s0022-2836(83)80101-6. [DOI] [PubMed] [Google Scholar]
- 30.Daar I O, Maquat L E. Mol Cell Biol. 1988;8:802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson B H, Taylor J, Kahler S G, Kirkman H N. Eur J Pediatr. 1981;136:35–39. doi: 10.1007/BF00441708. [DOI] [PubMed] [Google Scholar]
- 32.Carothers D J, Raefsky-Estrin C, Pons G, Patel M S. Arch Biochem Biophys. 1987;256:597–605. doi: 10.1016/0003-9861(87)90617-5. [DOI] [PubMed] [Google Scholar]
- 33.Beck T, Lloyd J B, Griffiths A. J Anat. 1967;101:467–478. [PMC free article] [PubMed] [Google Scholar]
- 34.Smith D E, Gridley T. Development (Cambridge, U.K.) 1992;116:555–561. doi: 10.1242/dev.116.3.555. [DOI] [PubMed] [Google Scholar]
- 35.Carothers D J, Pons G, Patel M S. Biochem J. 1988;249:897–902. doi: 10.1042/bj2490897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piko L, Matsumoto L. Dev Biol. 1976;49:1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- 37.Snow M H L. CIBA Found Symp. 1976;40:53–70. [Google Scholar]
- 38.Xia P, Rutledge J, Armstrong D T. Animal Reprod Sci. 1995;38:155–165. [Google Scholar]
- 39.Krehbiel R H. Physiol Zool. 1937;10:212–234. [Google Scholar]
- 40.Parr M B, Parr E L. Biol Reprod. 1986;34:393–403. doi: 10.1095/biolreprod34.2.393. [DOI] [PubMed] [Google Scholar]
- 41.Houghton F D, Thompsom J G, Kennedy C J, Leese H J. Mol Reprod Dev. 1996;44:476–485. doi: 10.1002/(SICI)1098-2795(199608)44:4<476::AID-MRD7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]