Abstract
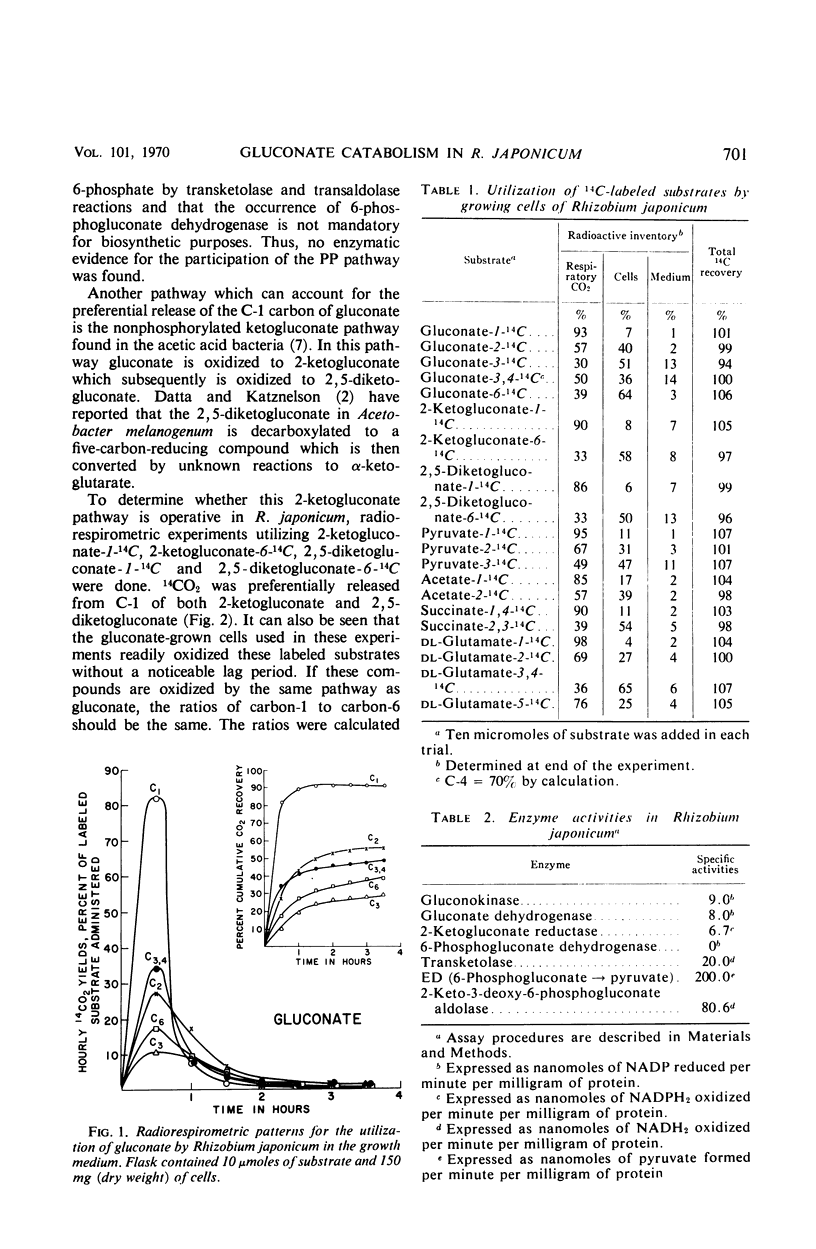
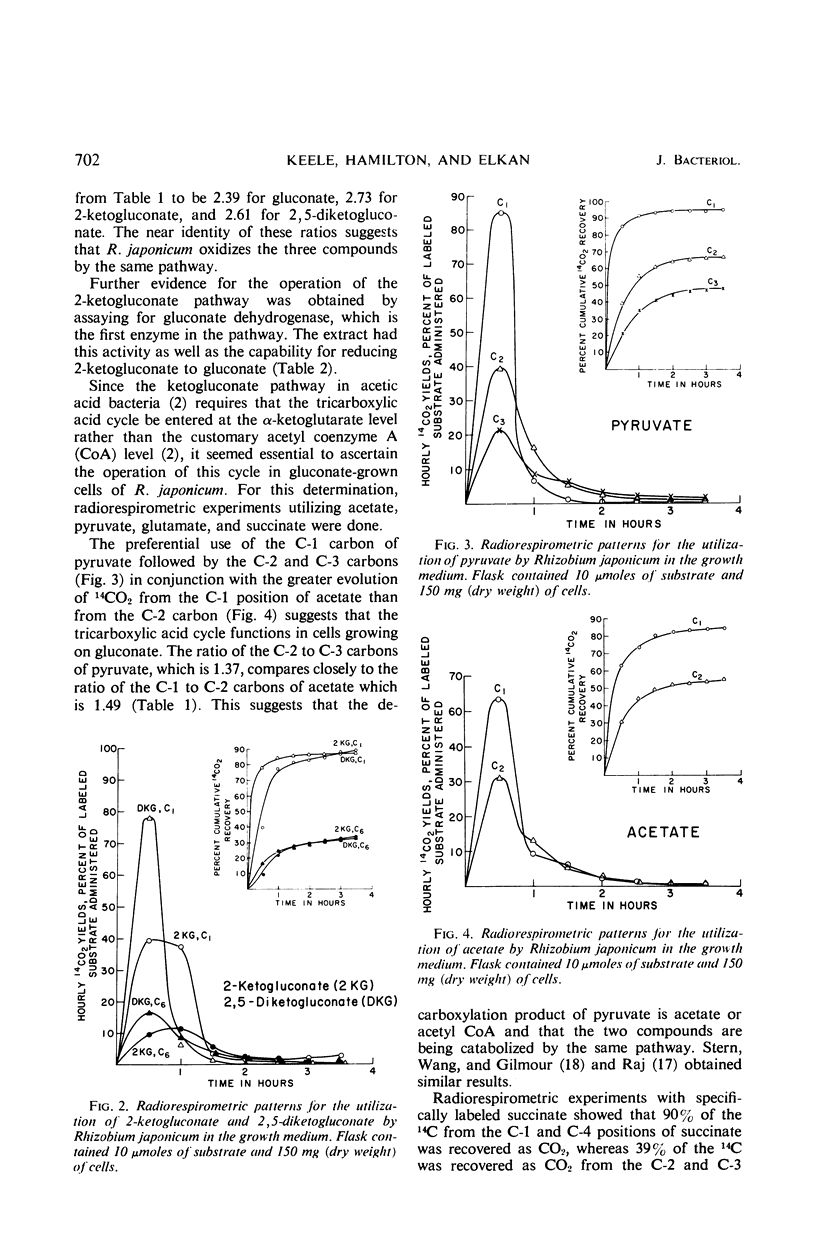
Gluconate catabolism in Rhizobium japonicum ATCC 10324 was investigated by the radiorespirometric method and by assaying for key enzymes of the major energy-yielding pathways. Specifically labeled gluconate gave the following results for growing cells, with values expressed as per cent 14CO2 evolution: C-1 = 93%, C-2 = 57%, C-3 = 30%, C-4 = 70%, C-6 = 39%. The preferential release of 14CO2 from C-1 and C-4 indicate that gluconate is degraded primarily by the Entner-Doudoroff pathway but the inequalities between C-1 and C-4 and between C-3 and C-6 indicate that another pathway(s) also participates. The presence of gluconokinase and a system for converting 6-phosphogluconate to pyruvate also indicate a role for the Entner-Doudoroff pathway. The extraordinarily high yield of 14CO2 from C-1 labeled gluconate suggests that the other participating pathway is a C-1 decarboxylative pathway. The key enzyme of the pentose phosphate pathway, 6-phosphogluconate dehydrogenase, could not be demonstrated. Specifically labeled 2-ketogluconate and 2,5-diketogluconate were oxidized by gluconate grown cells and gave ratios of C-1 to C-6 of 2.73 and 2.61, respectively. These compare with a ratio of 2.39 obtained with specifically labeled gluconate. Gluconate dehydrogenase, the first enzyme in the ketogluconate pathway found in acetic acid bacteria, was found. Oxidation of specifically labeled pyruvate, acetate, succinate, and glutamate by gluconate-grown cells yielded the preferential rates of 14CO2 evolution expected from the operation of the tricarboxylic acid cycle. These data are consistent with the operation of the Entner-Doudoroff pathway and tricarboxylic acid cycle as the primary pathways of gluconate oxidation in R. japonicum. An ancillary pathway for the initial breakdown of gluconate would appear to be the ketogluconate pathway which enters the tricarboxylic acid cycle at α-ketoglutarate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DATTA A. G., KATZNELSON H. Oxidation of 2, 5-diketogluconate by a cell-free enzyme preparation from Acetobacter melanogenum. Nature. 1957 Jan 19;179(4551):153–154. doi: 10.1038/179153a0. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., WANG C. H. Dissimilation of glucose and gluconic acid by Pseudomonas natriegens. J Bacteriol. 1962 Apr;83:879–886. doi: 10.1128/jb.83.4.879-886.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODDARD J. L., SOKATCH J. R. 2-KETOGLUCONATE FERMENTATION BY STREPTOCOCCUS FAECALIS. J Bacteriol. 1964 Apr;87:844–851. doi: 10.1128/jb.87.4.844-851.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D. C. The bacteroids of the genus Rhizobium. Bacteriol Rev. 1962 Jun;26:119–141. [PMC free article] [PubMed] [Google Scholar]
- Johnson G. V., Evans H. J., Ching T. Enzymes of the glyoxylate cycle in rhizobia and nodules of legumes. Plant Physiol. 1966 Oct;41(8):1330–1336. doi: 10.1104/pp.41.8.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZNELSON H. Production of pyruvate from 6-phosphogluconate by bacterial plant pathogens and legume bacteria. Nature. 1955 Mar 26;175(4456):551–552. doi: 10.1038/175551a0. [DOI] [PubMed] [Google Scholar]
- KATZNELSON H., TANENBAUM S. W., TATUM E. L. Glucose, gluconate, and 2-ketogluconate oxidation by Acetobacter melanogenum. J Biol Chem. 1953 Sep;204(1):43–59. [PubMed] [Google Scholar]
- KATZNELSON H., ZAGALLO A. C. Metabolism of rhizobia in relation to effectiveness. Can J Microbiol. 1957 Oct;3(6):879–884. doi: 10.1139/m57-097. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTENSON L. E., HAMILTON P. B., WILSON P. W. Dissimilation of 6-phosphogluconate by Azotobacter vinelandii. Biochim Biophys Acta. 1955 Feb;16(2):238–244. doi: 10.1016/0006-3002(55)90209-2. [DOI] [PubMed] [Google Scholar]
- Raj H. D. Radiorespirometric studies of Leucothrix mucor. J Bacteriol. 1967 Sep;94(3):615–623. doi: 10.1128/jb.94.3.615-623.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN I. J., WANG C. H., GILMOUR C. M. Comparative catabolism of carbohydrates in Pseudomonas species. J Bacteriol. 1960 Apr;79:601–611. doi: 10.1128/jb.79.4.601-611.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. H., STERN I. J., GILMOUR C. M. The catabolism of glucose and gluconate in Pseudomonas species. Arch Biochem Biophys. 1959 Apr;81(2):489–492. doi: 10.1016/0003-9861(59)90229-2. [DOI] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]


