Abstract
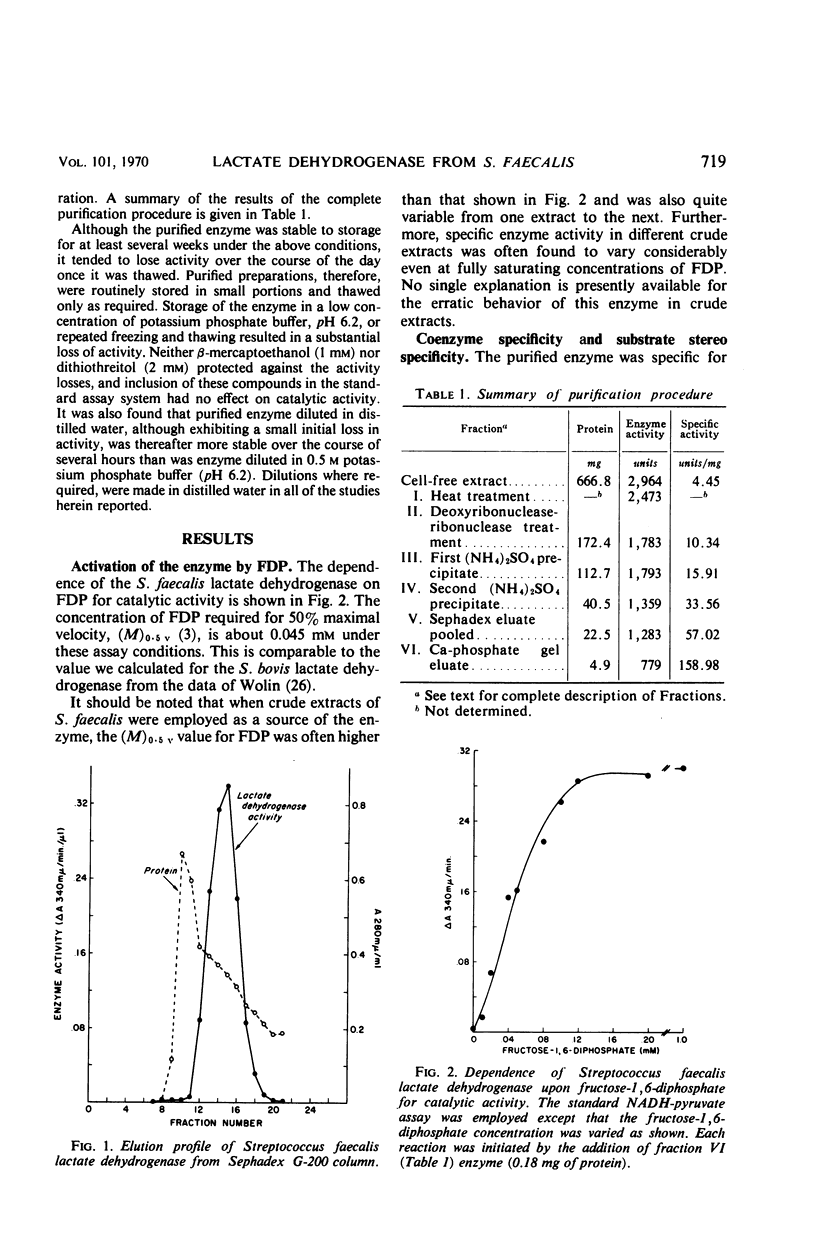
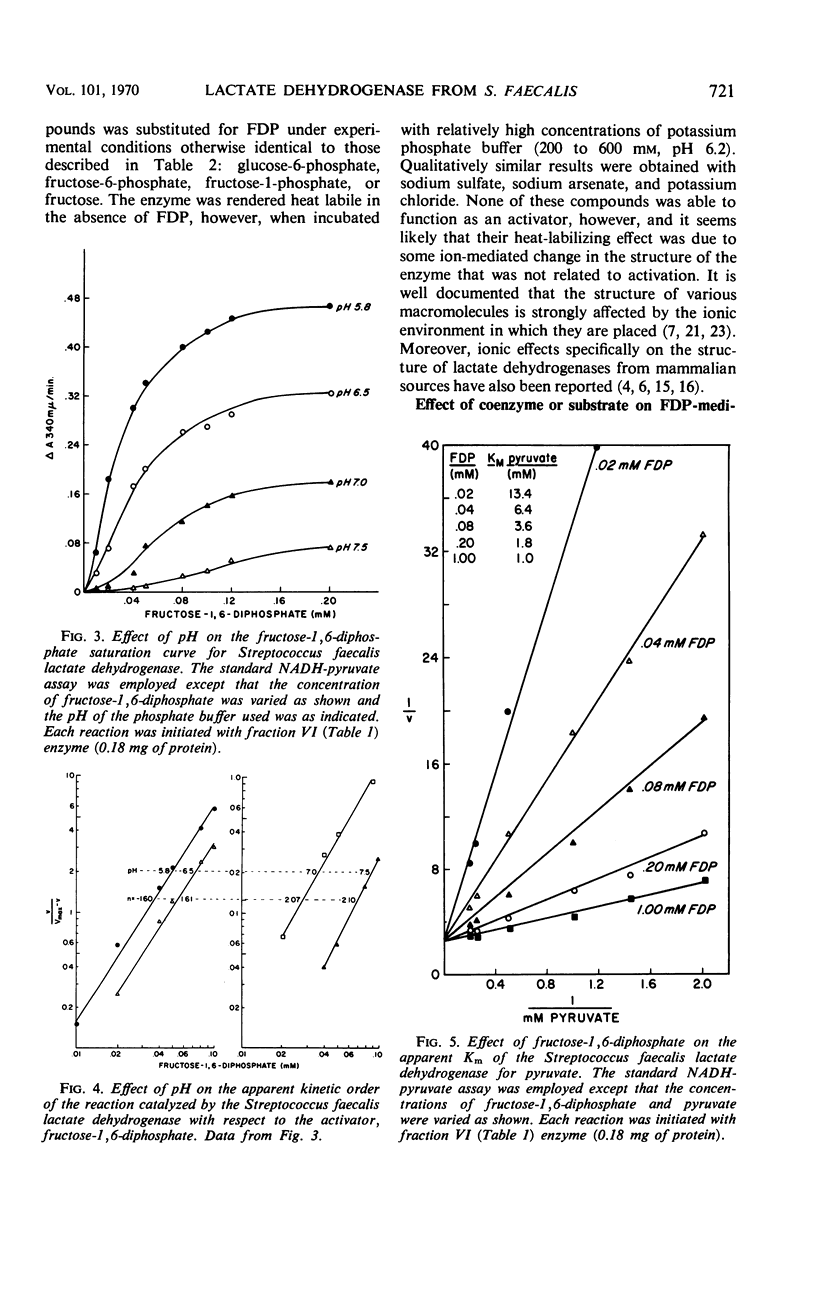
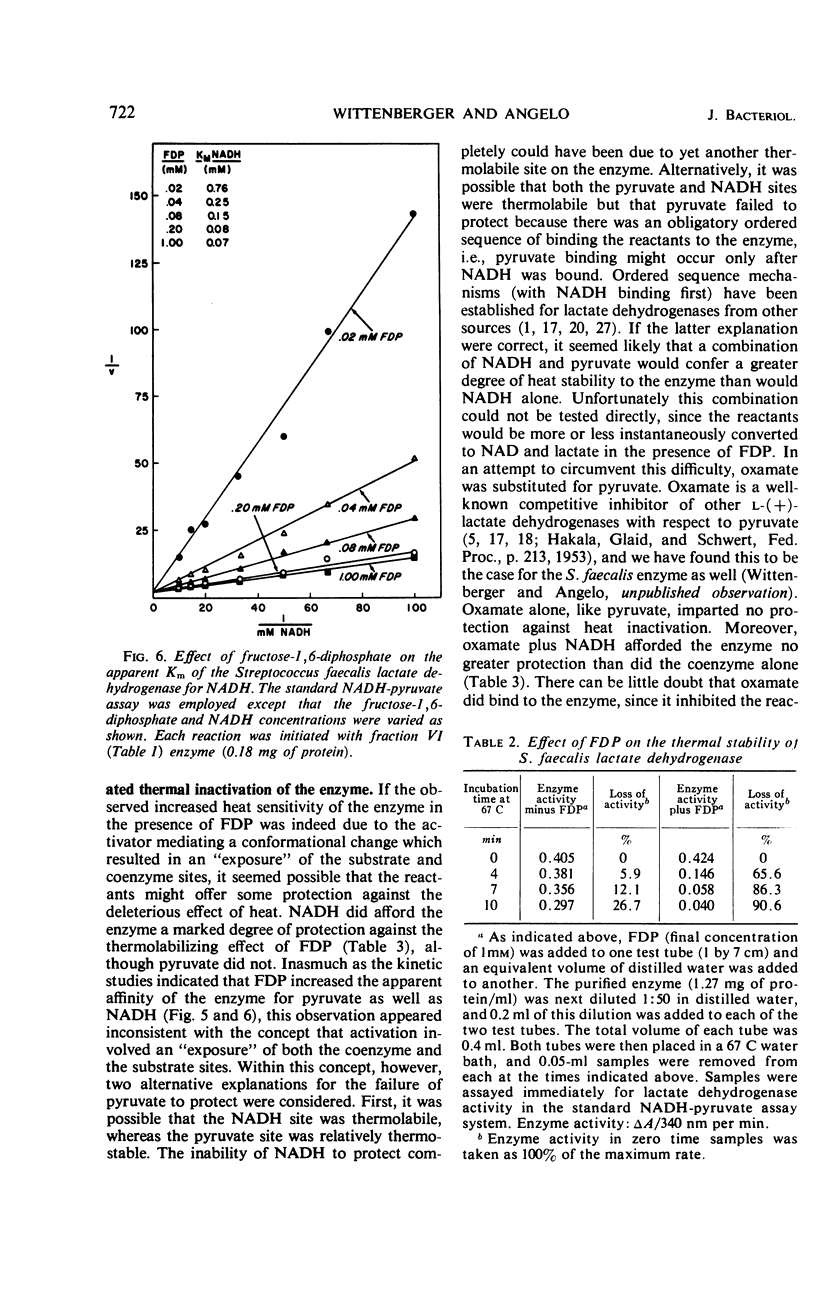
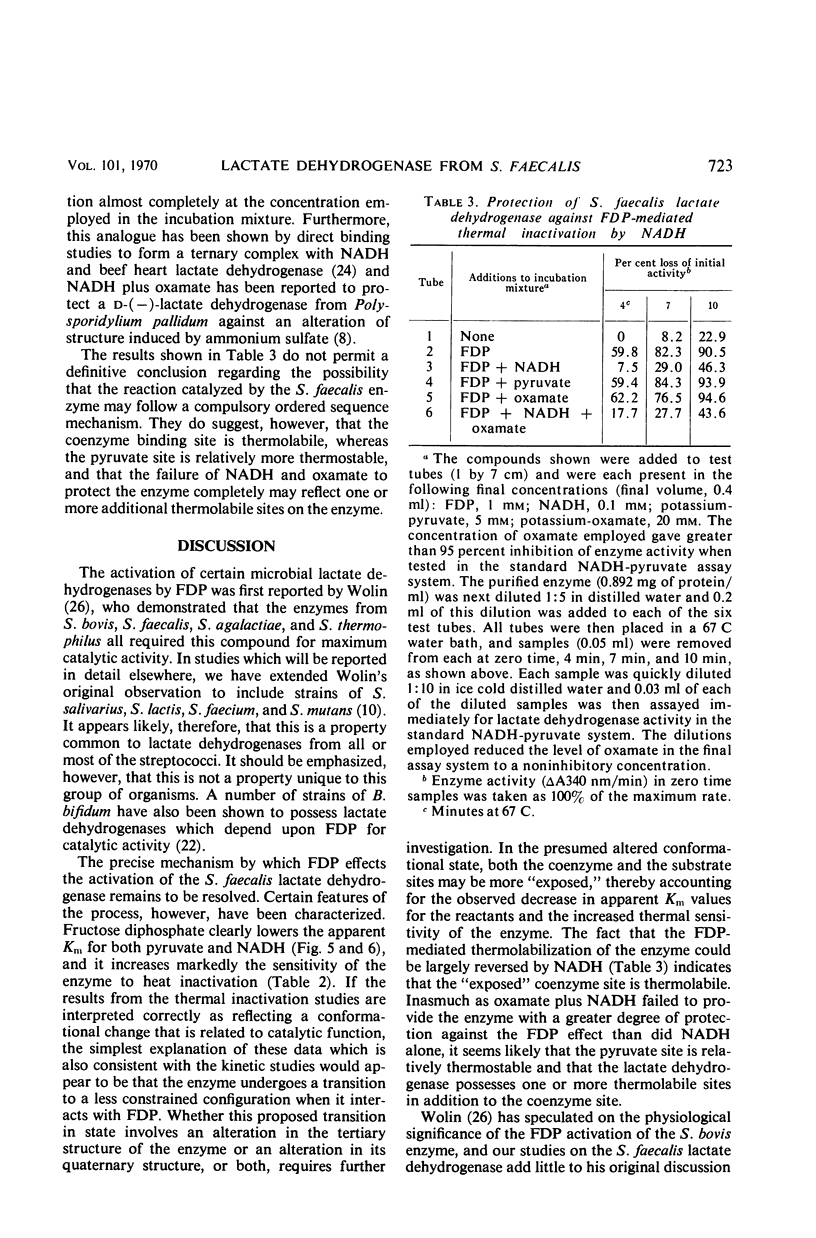
An l-(+)-lactate dehydrogenase was purified approximately 35-fold from crude extracts of Streptococcus faecalis. The purified enzyme had an absolute and specific requirement for fructose-1,6-diphosphate (FDP) for catalytic activity. The concentration of FDP required for 50% maximal activity was about 0.045 mm. The activator was bound to the enzyme more effectively at pH 5.8 than it was at a neutral or alkaline pH. Activation appeared to involve a conformational change in the enzyme which made the substrate and coenzyme sites more accessible to the respective reactants. Among the evidence supporting this hypothesis was the fact that FDP lowered significantly the apparent Km for both pyruvate and reduced nicotinamide adenine dinucleotide. Moreover, the enzyme, which was quite heat stable in the absence of any of the reactants, was rendered heat labile by FDP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON S. R., FLORINI J. R., VESTLING C. S. RAT LIVER LACTATE DEHYDROGENASE. 3. KINETICS AND SPECIFICITY. J Biol Chem. 1964 Sep;239:2991–2997. [PubMed] [Google Scholar]
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES: EFFECTORS FOR YEAST PHOSPHOFRUCTOKINASE DO NOT ALTER THE APPARENT KINETIC ORDER OF THE REACTION. Biochem Biophys Res Commun. 1965 Jan 4;18:1–5. doi: 10.1016/0006-291x(65)90872-7. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Kitto G. B., Pudles J., Kaplan N. O. Reversible inactivation of dehydrogenases. J Biol Chem. 1966 May 25;241(10):2431–2445. [PubMed] [Google Scholar]
- DENNIS D., KAPLAN N. O. D- and L-lactic acid dehydrogenases in Lactobacillus plantarum. J Biol Chem. 1960 Mar;235:810–818. [PubMed] [Google Scholar]
- DISABATO G., KAPLAN N. O. THE DENATURATION OF LACTIC DEHYDROGENASES. II. THE EFFECT OF UREA AND SALTS. J Biol Chem. 1965 Mar;240:1072–1076. [PubMed] [Google Scholar]
- FRIDOVICH I. Inhibition of acetoacetic decarboxylase by anions. The Hofmeister lyotropic series. J Biol Chem. 1963 Feb;238:592–598. [PubMed] [Google Scholar]
- Garland R. C., Kaplan N. O. Salt-induced alteration of D(-) lactate dehydrogenase from Polyspondylium pallidum. Biochem Biophys Res Commun. 1967 Mar 21;26(6):679–685. doi: 10.1016/s0006-291x(67)80126-8. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus. II. Evidence for allosteric inhibition of an inducible malate dehydrogenase (decarboxylating) by ATP and glycolytic intermediate products. Biochim Biophys Acta. 1969 Apr 22;178(2):205–212. doi: 10.1016/0005-2744(69)90390-8. [DOI] [PubMed] [Google Scholar]
- London J. Regulation and function of lactate oxidation in Streptococcus faecium. J Bacteriol. 1968 Apr;95(4):1380–1387. doi: 10.1128/jb.95.4.1380-1387.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert C. L., Massaro E. J. In vitro hybridization of lactate dehydrogenase in the presence of arsenate and nitrate lons. Arch Biochem Biophys. 1966 Aug;115(2):417–426. doi: 10.1016/0003-9861(66)90291-8. [DOI] [PubMed] [Google Scholar]
- Markert C. L., Massaro E. J. Lactate dehydrogenase isozymes: dissociation and denaturation by dilution. Science. 1968 Nov 8;162(3854):695–697. doi: 10.1126/science.162.3854.695. [DOI] [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- PAPACONSTANTINOU J., COLOWICK S. P. The role of glycolysis in the growth of tumor cells. I. Effects of oxamic acid on the metabolism of Ehrlich ascites tumor cells in vitro. J Biol Chem. 1961 Feb;236:278–284. [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. Lactic dehydrogenase. III. Mechanism of the reaction. J Biol Chem. 1956 Nov;223(1):157–170. [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- VONHIPPEL P. H., WONG K. Y. NEUTRAL SALTS: THE GENERALITY OF THEIR EFFECTS ON THE STABILITY OF MACROMOLECULAR CONFORMATIONS. Science. 1964 Aug 7;145(3632):577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Warren J. C., Stowring L., Morales M. F. The effect of structure-disrupting ions on the activity of myosin and other enzymes. J Biol Chem. 1966 Jan 25;241(2):309–316. [PubMed] [Google Scholar]
- Wittenbe C. L. Unusual kinetic properties of a DPN-linked lactate dehydrogenase from Butyribacterium rettgeri. Biochem Biophys Res Commun. 1966 Mar 22;22(6):729–736. doi: 10.1016/0006-291x(66)90209-9. [DOI] [PubMed] [Google Scholar]
- ZEWE V., FROMM H. J. KINETIC STUDIES OF RABBIT MUSCLE LACTATE DEHYDROGENASE. II. MECHANISM OF THE REACTION. Biochemistry. 1965 Apr;4:782–792. doi: 10.1021/bi00880a024. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stouthamer A. H. Fermentation of glucose, lactose, galactose, mannitol, and xylose by bifidobacteria. J Bacteriol. 1968 Aug;96(2):472–478. doi: 10.1128/jb.96.2.472-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


