Abstract
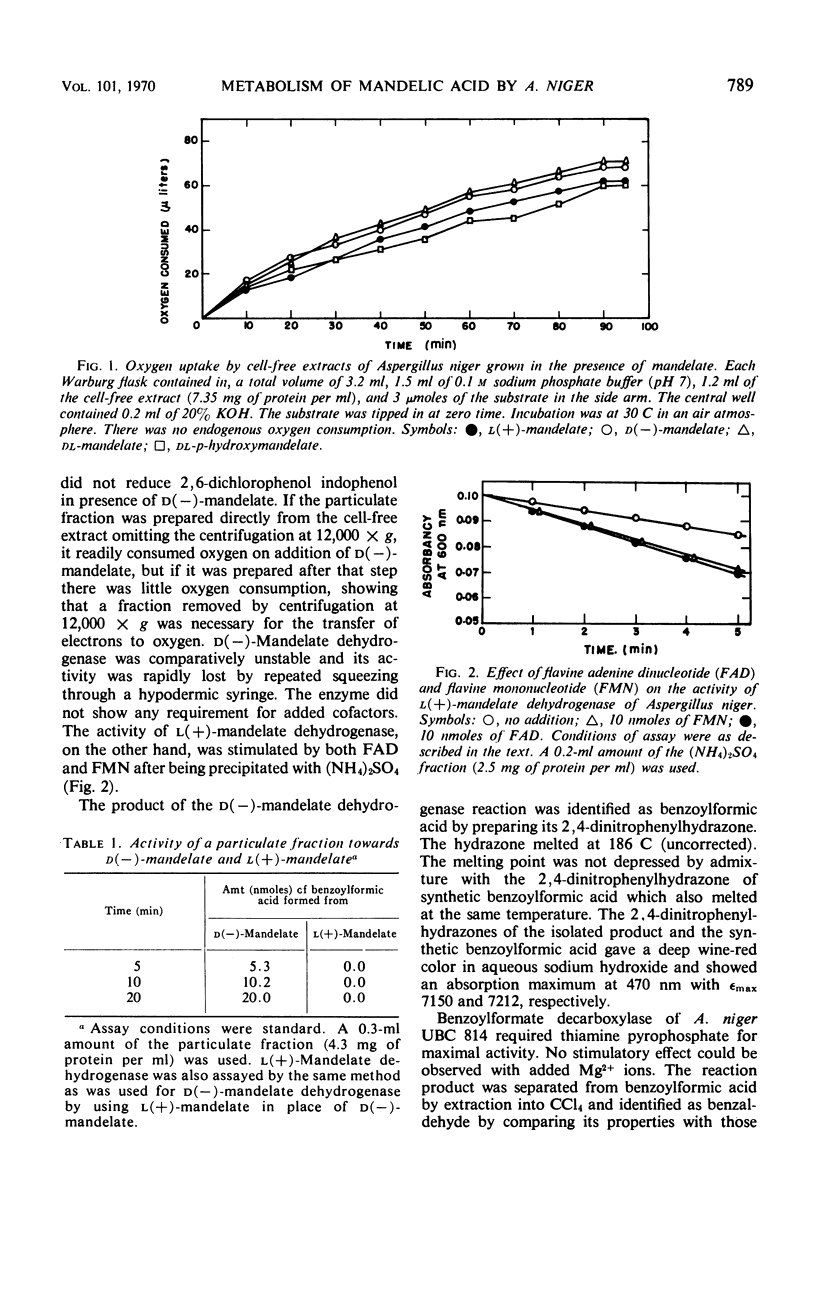
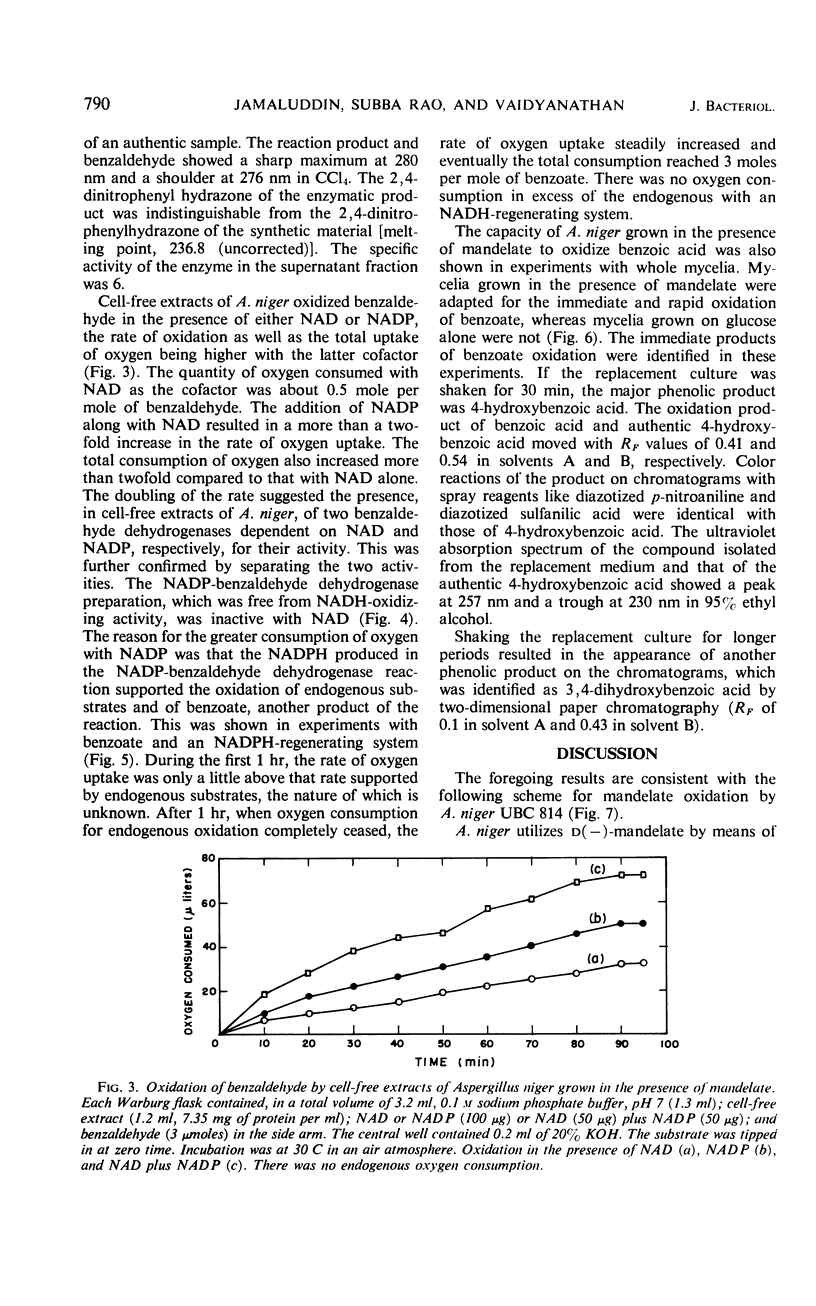
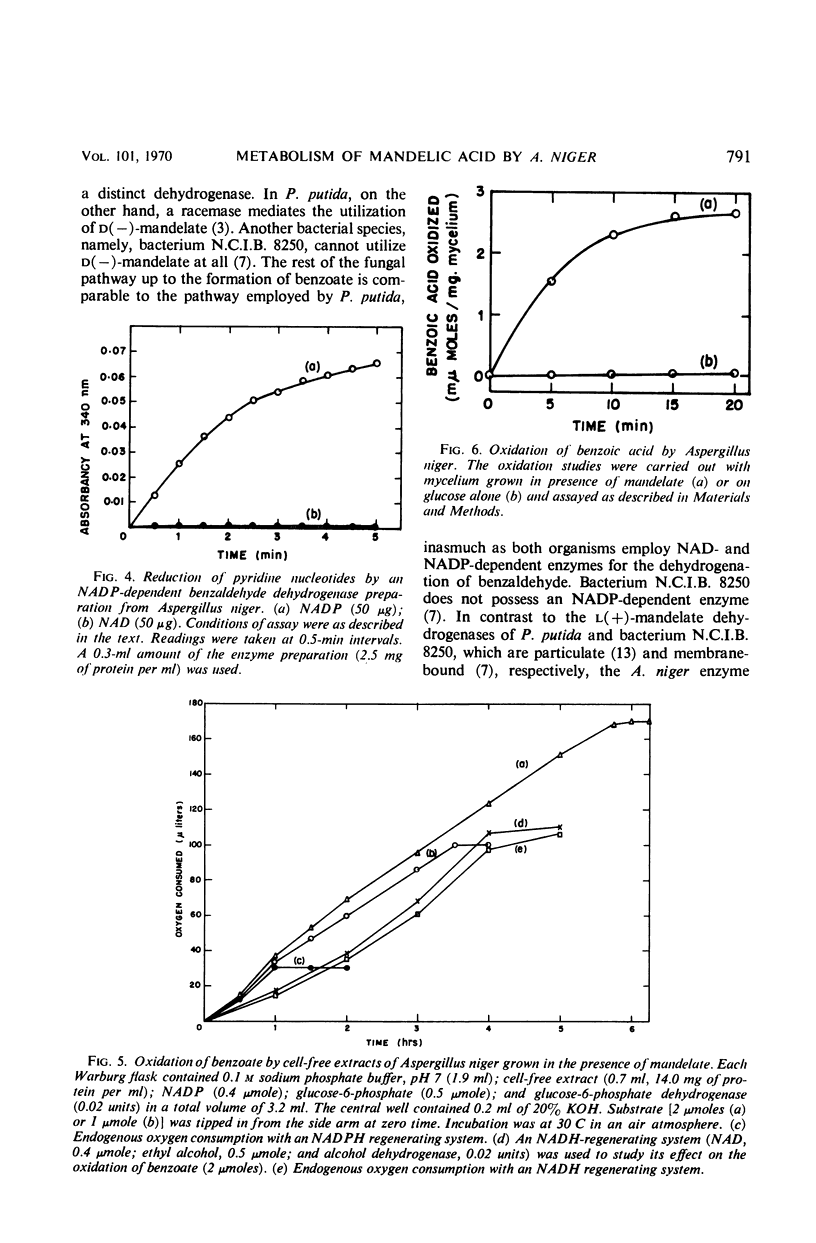
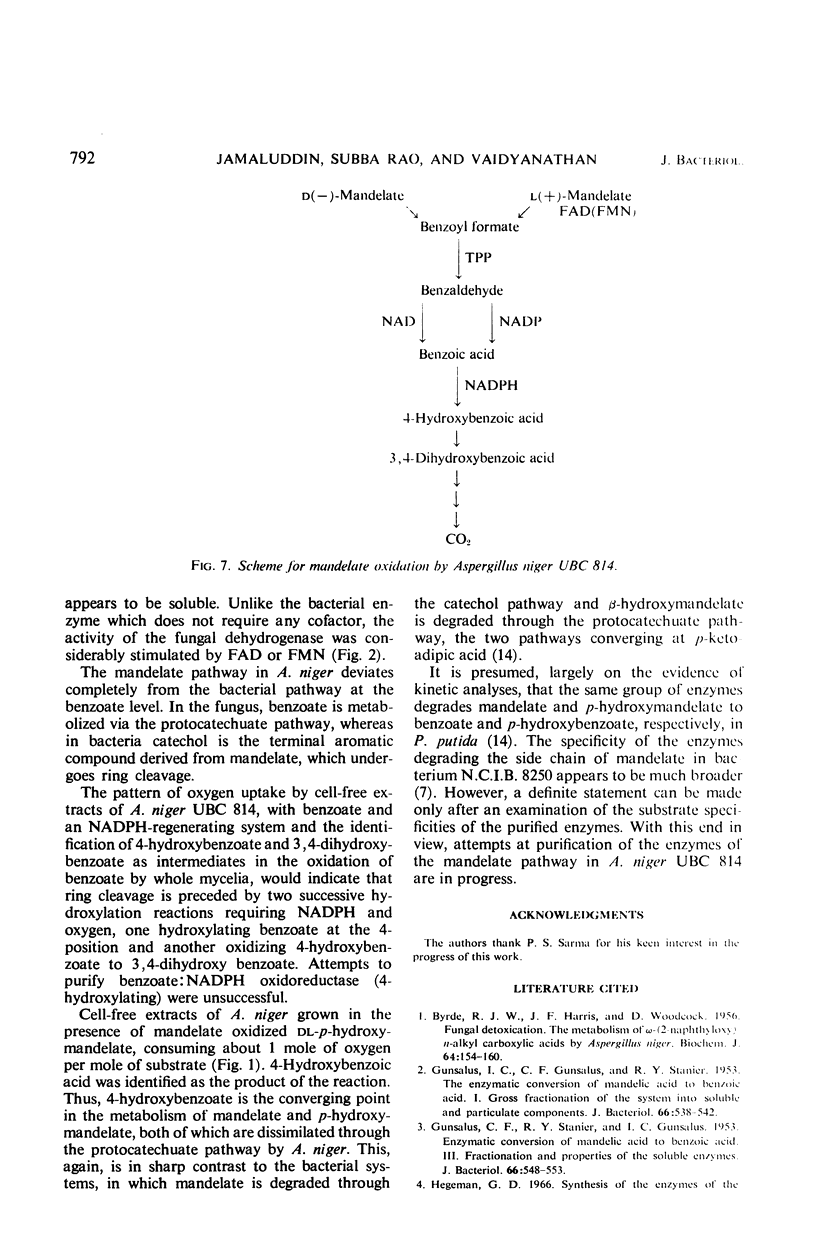
Cell-free extracts of Aspergillus niger UBC 814 grown in the presence of dl-mandelate oxidized both d(−)- and l(+)-mandelate via benzoylformate and benzaldehyde to benzoate. dl-p-Hydroxymandelate was oxidized, presumably through a parallel pathway, to p-hydroxybenzoate. A particulate d(−)-mandelate dehydrogenase and a supernatant fraction l(+)-mandelate dehydrogenase converted their respective substrates to benzoylformate. Both flavine adenine dinucleotide and flavine mononucleotide showed a stimulatory effect on the activity of the l(+)-mandelate dehydrogenase. Benzoylformate was decarboxylated to benzaldehyde by an enzyme requiring thiamine pyrophosphate for maximal activity. Two benzaldehyde dehydrogenases dependent on nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), respectively, for their activity dehydrogenated benzaldehyde to benzoate. In the presence of reduced NADP (NADPH), benzoate was oxidized via p-hydroxybenzoate and protocatechuate. Reduced NAD could not replace NADPH. Sensitive methods of assay for d(−)-mandelate dehydrogenase and benzoylformate decarboxylase are described. The fungal pathway is compared with these systems in bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GUNSALUS I. C., GUNSALUS C. F., STANIER R. Y. The enzymatic conversion of mandelic acid to benzoic acid. I. Gross fractionation of the system into soluble and particulate components. J Bacteriol. 1953 Nov;66(5):538–542. doi: 10.1128/jb.66.5.538-542.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. 3. Isolation and properties of constitutive mutants. J Bacteriol. 1966 Mar;91(3):1161–1167. doi: 10.1128/jb.91.3.1161-1167.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUYVER A. J., VAN ZIJP J. C. The production of homogentisic acid out of phenylacetic acid by Aspergillus niger. Antonie Van Leeuwenhoek. 1951;17(5):315–324. doi: 10.1007/BF02062278. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Metabolism of mandelate and related compounds by bacterium NCIB 8250. J Gen Microbiol. 1968 Sep;53(2):259–273. doi: 10.1099/00221287-53-2-259. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., Towers G. H. Degradation of aromatic amino acids by fungi. I. Fate of L-phenylalanine in Schizophyllum commune. Can J Biochem. 1967 Nov;45(11):1659–1665. doi: 10.1139/o67-196. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., GUNSALUS I. C., GUNSALUS C. F. The enzymatic conversion of mandelic acid to benzoic acid. II. Properties of the particulate fractions. J Bacteriol. 1953 Nov;66(5):543–547. doi: 10.1128/jb.66.5.543-547.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]



