Abstract
The development of two major subdivisions of the vertebrate nervous system, the midbrain and the cerebellum, is controlled by signals emanating from a constriction in the neural primordium called the midbrain/hindbrain organizer (Joyner, A. L. (1996) Trends Genet. 12, 15–201). The closely related transcription factors Pax-2 and Pax-5 exhibit an overlapping expression pattern very early in the developing midbrain/hindbrain junction. Experiments carried out in fish (Krauss, S., Maden, M., Holder, N. & Wilson, S. W. (1992) Nature (London) 360, 87–89) with neutralizing antibodies against Pax-b, the orthologue of Pax-2 in mouse, placed this gene family in an regulatory cascade necessary for the development of the midbrain and the cerebellum. The targeted mutation of Pax-5 has been reported to have only slight effects in the development of structures derived from the isthmic constriction, whereas the Pax-2 null mutant mice show a background-dependent phenotype with varying penetrance. To test a possible redundant function between Pax-2 and Pax-5 we analyzed the brain phenotypes of mice expressing different dosages of both genes. Our results demonstrate a conserved biological function of both proteins in midbrain/hindbrain regionalization. Additionally, we show that one allele of Pax-2, but not Pax-5, is necessary and sufficient for midbrain and cerebellum development in C57BL/6 mice.
The neural tube is regionalized by successive division of the neuroepithelium into distinct areas of the central nervous system. The boundaries between these regions can be recognized by the region-specific expression of certain genes as well as by morphological landmarks like constrictions in the neural tube (1). The earliest four subdivisions of the developing neural tube are the forebrain, the midbrain, the hindbrain, and the spinal cord. The boundary between midbrain and hindbrain is of particular interest because it has been shown to control the development of the mesencephalon and metencephalon (reviewed in ref. 2). A number of regulatory proteins are known to play an important role in the establishment and the maintenance of the midbrain/hindbrain organizer, among them the two secreted molecules Wnt-1 and FGF-8, the homeodomain containing transcription factors En-1 and En-2, and the paired domain-containing transcription factors Pax-2 and Pax-5 (refs. 3–9; reviewed in ref. 10). Pax-2 is the earliest of these genes expressed in the prospective midbrain/hindbrain boundary, and its expression precedes the above-mentioned genes (11). The functions of all these molecules have been studied in mice (3, 4, 6–9, 12, 13) by means of targeted or naturally occurring mutations. The phenotypes described for these mutations include either abnormalities in the development of the structures derived from the organizing center (En-2 and Pax-5) or variable deletions of midbrain and cerebellum tissue around the mesencephalon/metencephalon constriction (Wnt-1 and En-1). It has been demonstrated that the weak brain phenotype of the En-2 null mutation is caused by functional redundancy of a second member of this gene family, En-1, which is overlappingly expressed (14). A similar mechanism of functional substitution in this region has been suggested to explain the weak brain phenotype of Pax-5 (3) because of redundant function of the closely related Pax-2 gene.
Furthermore, cooperation of Pax-2 and Pax-5 in midbrain and cerebellum development has been suggested on the basis of the phenotype of mice carrying both the Pax-5 and Krd mutations (15). The Krd mutation results from a mostly uncharacterized 7-cM deletion in chromosome 19, which comprises among other alterations the deletion of the whole Pax-2 locus (16). The homozygous Krd mutation is lethal at the preimplantation stage. The compound Pax-5 homozygous and Krd heterozygous mutant mice show a complete loss of the posterior midbrain and cerebellum (15), which is similar to the phenotype of the En-1 mutant mice (13).
In the present study we analyze the midbrain/hindbrain phenotype of mice carrying a targeted deletion of Pax-2 (back-crossed into C57BL/6) and discover that it has no obvious morphological alterations in the brainstem, suggesting a possible redundant function by Pax-5 because of the partially overlapping expression in this region. To prove this point we have performed heterozygous intercrosses between Pax-2 and Pax-5 knockout mice to precisely alter and subsequently determine the critical Pax gene dosage necessary for the development of the midbrain and cerebellum in vivo. We demonstrate that one allele of Pax-2, but not Pax-5, is sufficient for normal patterning of the midbrain and cerebellum. Furthermore, we show that Pax-2 expression does not change in the Pax-5 null mutant and vice versa, suggesting that these genes are not subjected to cross-regulation by each other. In addition, we document, by cresyl violet staining and radioactive in situ hybridization to En-1, that double homozygous Pax-2 and Pax-5 mice show an alteration of the midbrain tectum and the caudal midbrain tegmentum as well as a complete loss of the cerebellum. Taken together, our data argue for the functional substitution between Pax-2 and Pax-5 in brain regionalization.
MATERIALS AND METHODS
Mice.
Pax-2 (+/−) mutant mice bred on the C57BL/6J and 129/Sv hybrid background (sixth generation C57BL/6J) were crossed with Pax-5 mutant mice (15) bred to a C57BL/6J/129/Sv hybrid background. The respective genotypes of the crossed mice were determined by genomic Southern blot by using the previously reported DNA fragments for Pax-2 (17) and Pax-5 (3).
Histological Analysis.
Embryos were dissected out, washed in PBS for 10 min, and fixed overnight at 4°C in 4% paraformaldehyde. Subsequently, embryos were washed again in PBS and then processed for paraffine sectioning. Sections (8–10 μm thick) were stained with cresyl violet and photographed by using the Zeiss Axiophot.
Whole-Mount in Situ Hybridization Analysis.
Whole-mount in situ hybridization was performed as described in ref. 18. Single-stranded RNA molecules were labeled with digoxigenin-UTP (Boehringer Mannheim), after hybridization the labeled RNA probes were detected with an antidigoxigenin antibody coupled to alkaline phophatase.
Otx-2, Wnt-1, and En-2 have been described previously (19). The Fgf-8 probe was a −400-bp fragment corresponding to isoform4 and was kindly provided by Gail Martin (University of California at San Francisco).
Radioactive in Situ Hybridization.
In situ hybridization experiments on sections were performed as previously described by using 35S-labeled RNA probes. The Pax-2, Pax-5, Pax-7, and Otx-2 probes have been described previously (17, 19).
RESULTS
The Previously Described Brainstem Alterations in the Pax-2 Null Mutant Mice Are a Consequence of the 129/Sv Inbred Background.
The previously described Pax-2 knockout mouse (20) has been reported to show exencephaly caused by a failure of the neural tube to close at the midbrain level (9). This phenotype occurred in 100% of the homozygous animals and also in a low percentage in the heterozygous animals dependent on the background. The exencephaly was constant between the animals and did not affect the expression of Wnt-1, Pax-5, and En-1, markers for the midbrain/hindbrain junction at embryonic day 9.5 (e9.5), suggesting no tissue loss of either the midbrain or the cerebellar primordium (9). Examination of marker gene expression at later stages was not possible because of the gross morphological brainstem alterations. However, midbrain and cerebellar tissue clearly could be identified in cresyl violet-stained sagittal e13 and e16 homozygous exencephalic embryos (this study; data not shown). To clarify if the exencephaly was a direct result of the Pax-2 mutation or a consequence of the genetic background, we crossed the Pax-2 129/Sv inbred mice into C57 BL/6 mice for at least six generations. We then performed heterozygous intercrosses to obtain mice carrying a null allele of the Pax-2 locus. The resulting Pax-2 homozygous animals show a lower frequency of exencephaly (30%) as indicated in Table 1. However, 70% of the animals did not show morphological alterations by gross inspections. None of the heterozygous animals obtained exhibited exencephaly.
Table 1.
Genetic background-dependent exencephaly in the Pax-2 mutant
| Mice | 129sv (n = 122)
|
C57B6 (n = 184)
|
||
|---|---|---|---|---|
| Normal | Exencephalus | Normal | Exencephalus | |
| wt | 100% | 0% | 100% | 0% |
| −/+ | 100% | 0% | 100% | 0% |
| −/− | 0% | 100% | 70% | 30% |
All of the homozygous mice of the 129sv strain showed exencephaly, but none of the heterozygous or the wild-type ones did so. In the mutants backcrossed into the C57BL/6 strain, the percentage of exencephaly in the homozygous animals decreased to 30%. The offspring of heterozygous crosses was genotyped and analyzed at different stages of development. All the cases of exencephaly were immediately observable as severe morphological alterations. wt, wild type.
The Pax-2 Mutant Mouse Does Not Show Midbrain/Hindbrain Patterning Defects as Demonstrated by Morphological Appearance and Marker Gene Expression During Embryonic Development.
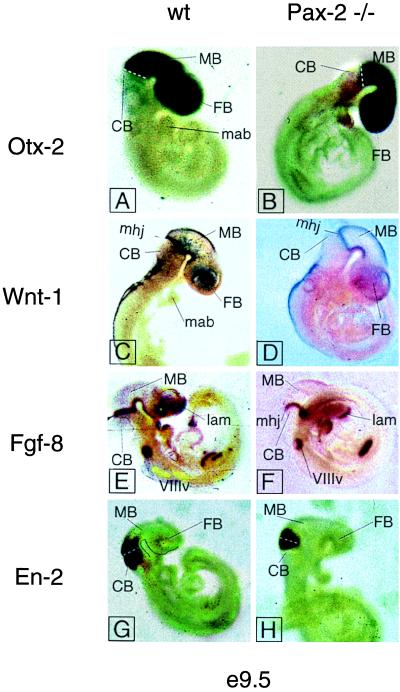
To assess a possible alteration of midbrain/hindbrain marker gene expression in the Pax-2 null mutant we performed whole-mount in situ hybridization at the level of the midbrain/hindbrain junction. We used Fgf-8 and Wnt-1 probes to visualize a possible effect on the isthmic organizer in the Pax-2 mutant mouse (21). As can be seen in Fig. 1 neither Fgf-8 nor Wnt-1 expression patterns change as compared with the wild-type animals. We conclude that the lack of a midbrain/hindbrain phenotype is caused by the functional rescue by the closely related and overlappingly expressed gene Pax-5. To further investigate the possibility of early tissue loss in the midbrain and cerebellar anlagen, we performed whole-mount in situ hybridization for Otx-2 and En-2 (22). Both Otx-2 and En-2 are markers for the midbrain and/or cerebellum (10, 19, 23). The territory of expression of Otx-2 includes the forebrain and midbrain, with a sharp caudal boundary at the isthmic constriction (19, 23). Thus Otx-2 can be used as a marker for the midbrain. Besides labeling the caudal midbrain, En-2 also is expressed in the cerebellar primordium (10, 22). The same results were obtained by whole-mount in situ hybridization with the En-2, Wnt-1, and Fgf-8 probes on Pax-2 knockout embryos at e10.5.
Figure 1.
Whole-mount in situ hybridization of early midbrain-hindbrain junction markers in wild-type and Pax-2 −/− e9.5 mouse embryos. None of the markers shows alteration in the mutant as compared with the wild type. The white dotted line in A, B, G, and H, and the arrowhead in E mark the isthmic constriction. The black line in G follows the ventral side of the forebrain and midbrain. CB, cerebellum; FB, forebrain; mab, mandibular part of the first branchial arch; MB, midbrain; mhj, midbrain-hindbrain junction; VIIIv, otic vesicle; lam, lamina terminalis.
Additionally, we performed a detailed morphological study of serially sectioned, cresyl violet-stained brains of Pax-2 mutant embryos at e14. No morphological alterations could be detected as compared with the wild type (not shown). All the regions reported to have strong Pax-2 expression (17), e.g., the nucleus ambiguous, cerebellar nuclei, and the reticular formation appeared normal by morphological inspection. Furthermore, expression of En-1 was normal in the Pax-2 null mutants (Fig. 2). However, we cannot rule out minor alterations in the cell composition of specific nuclei. The development of the cerebellum could not be studied in depth in the Pax-2 homozygous mice because the embryos died around the time of birth because of the absence of kidneys (20).
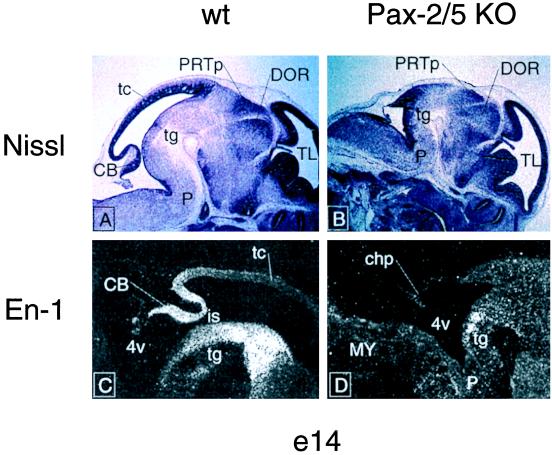
Figure 2.
In situ hybridization of midbrain-hindbrain junction markers on sagittal sections of Pax-2 −/−, Pax-5 −/−, and wild-type e14 mouse embryos. None of the marker expression patterns shows variation in the mutants (C57BL/6) as compared with wild type. 4v, fourth ventricle; CB, cerebellum; tc, midbrain tectum; tg, midbrain tegmentum; is, isthmus constriction. Neither Pax-2 in the Pax-2 mutant nor Pax-5 in the Pax-5 mutant are shown (gray squares).
Pax-2 and Pax-5 Expression Overlaps in the Midbrain/Hindbrain Junction.
To postulate functional substitution between two proteins in a specific brain region, it is necessary that both genes are overlappingly expressed (temporally and spatially) in that particular region. We tested for a possible overlapping expression of Pax-2 and Pax-5 at the level of the midbrain/hindbrain junction in wild-type animals by in situ hybridization that used radiolabeled probes on sections of e14 embryo brains (Fig. 2). We found that both genes were expressed at the midbrain/hindbrain junction demarcated by the rostral border of Otx-2 expression (Fig. 2) (23). En-1, a crucial marker gene for isthmic derivatives (13), is not altered in the mutants (Fig. 2).
To rule out a possible cross-regulation between Pax-2 and Pax-5 in this region we analyzed the expression of Pax-2 in the Pax-5 homozygous mutant mouse and vice versa by means of radioactive in situ hybridization. As shown in Fig. 2, neither Pax-2 nor Pax-5 expression boundaries were altered in the mutants as compared with the wild type.
The double homozygous mutant mice completely lack presumptive midbrain tectum and cerebellum because of an early loss of organizer tissue as illustrated by β-galactosidase staining for the Pax-5 lac-Z allele at e8.5 (see Fig. 3). This result is the earliest loss of organizer tissue so far reported.
Figure 3.
Early loss of midbrain/hindbrain tissue in Pax-2/Pax-5 compound mutant mice. Early deletion of midbrain/hindbrain junction tissue shown by β-galactosidase staining in e8.5 mouse embryos (12 somites) carrying the Pax-5-lacZ allele. (A) The Pax-2 +/+ Pax-5 −/− embryo show normal expression of the Pax-5-lacZ allele in the midbrain/hindbrain junction. (B) The compound Pax-2/Pax-5 mutant is missing the tissue of the midbrain/hindbrain junction as shown by the lack of β-galactosidase staining. Arrowhead in A points to lacZ-expressing tissue. Arrowhead in B points to the presumptive midbrain/hindbrain junction region. The dotted lines follow the ventral side of the rostral neural tube. FB, forebrain; HB, hindbrain; MB, midbrain.
One Functional Pax-2 Allele Is Necessary and Sufficient for the Normal Development of the Midbrain and Cerebellum in the Absence of Pax-5.
To determine the Pax gene dosage necessary to ensure normal midbrain/hindbrain development in vivo we performed heterozygous intercrosses between Pax-2 and Pax-5 mutant animals. The double heterozygous animals did not show any morphological alterations (Table 2); they were fertile and bred according to Mendelian laws. Mice heterozygous for Pax-2 and homozygous for the Pax-5 mutation did not show brain alterations during embryonic development (see Table 2). Furthermore, they were born alive and survived for up to 2 weeks. These survivors were smaller than their wild-type littermates, looked runted, and their brains showed only minor morphological alterations as previously published for the Pax-5 (−/−) mice (data not shown). However, mice homozygous for the Pax-2 mutation and heterozygous for the Pax-5 mutation exhibited gross alterations of the midbrain and cerebellar primordium. Morphological inspection of animals homozygous for both the Pax-2 and the Pax-5 mutant alleles revealed the absence of cerebellum and midbrain tectum, as well as the caudal part of the tegmentum (Fig. 4). In agreement with this finding, the absence of En-1 (13) expression in this region, proven by in situ hybridization, confirmed the tissue loss around this region. The fact that the phenotypes of Pax-5 heterozygous, Pax-2 homozygous animals, and the compound Pax-2/Pax-5 homozygous mice both show the same alterations argues for a critical dosage of Pax proteins for normal development of the midbrain and cerebellum.
Table 2.
Effect of Pax-2 and Pax-5 dosage on prenatal brain morphology in the C57BL/6 strain
| Mouse strain c57/Bl6 | Brain morphology | Number of animals (n = 116) |
|---|---|---|
| Wild type | “Normal” | 9 |
| (approx. 1/16) | ||
| Pax-2 −/− | “Normal” | 7 |
| (30% exenceph.) | (approx. 1/16) | |
| Pax-5 −/− | “Normal” | 8 |
| (approx. 1/16) | ||
| Pax-2 +/− | “Normal” | 33 |
| Pax-5 +/− | (approx. 1/4) | |
| Pax-2 +/+ | “Normal” | 12 |
| Pax-5 +/− | (approx. 1/8) | |
| Pax-2 +/− | “Normal” | 14 |
| Pax-5 +/+ | (approx. 1/8) | |
| Pax-2 +/− | “Normal” | 14 |
| Pax-5 −/− | (approx. 1/8) | |
| Pax-2 −/− | Altered | 11 |
| Pax-5 +/− | (approx. 1/8) | |
| Pax-2 −/− | Altered | 8 |
| Pax-5 −/− | (approx. 1/16) |
Brain morphology was analyzed on histological sections of embryos of different ages stained with cresyl violet (Nissl method). Phenotypes showing no morphological alteration, or only subtle morphological alteration were scored as “normal.” Only major anatomical alterations, as the ones reported in this study (see text) were scored as “altered.” The Pax-5 homozygous mouse showed minor changes in the inferior colliculus and in the cerebellar foliation pattern only apparent at late stages of development, as reported (3), and therefore was entered in this table as “normal.”
Figure 4.
Major morphological alterations of the met/mesencephalon in the Pax-2/Pax-5 compound mutant. Sagittal sections of wild-type (A and C) and compound Pax-2/Pax-5 (B and D) e14 embryos stained with cresyl violet (Nissl; A and B) and with radioactive probe for Engrailed-1 (in situ hybridization; C and D). The compound Pax-2/Pax-5 mutant shows loss of the midbrain tectum and of part of the midbrain tegmentum, and complete loss of the cerebellar anlage. The results of the morphological analysis are confirmed by in situ hybridization by using the En-1 probe as a marker for the cerebellum and the caudal part of the tectum and the midbrain tegmentum. 4v, fourth ventricle; CB, cerebellum; chp, choroid plexus; DOR, dorsal thalamus; is, isthmus; MY, medulla; P, pons; PRTp, precommissural pretectum; tc, midbrain tectum; tg, midbrain tegmentum; TL, telencephalon.
DISCUSSION
Effect of the Background on the Phenotype.
The observation that the Pax-2 mutation in the C57/BL6 genetic background results in exencephaly only in a low percentage of cases suggests that there is no direct involvement of Pax-2 in controlling the closure of the neural folds in the midbrain region. In this context, it is interesting to note that a null mutation of the Pax-2 gene originally produced in another 129/Sv background results only in a low degree of exencephaly (12). The following alternatives might explain this discrepancy. First, the animals used in ref. 12 were of mixed genetic background (C57BL/6/C3Hx102 F1), resulting in a more heterogeneous genome, whereas the animals used in ref. 20 were isogenic 129/Sv. Any possible genomic instabilities from the pure 129/Sv cell line could be buffered in a more heterogeneous background. Second, most studies involving homologous recombination make use of embryonic stem (ES) cell lines derived from 129 strain mice. However, a high degree of genetic variation within the 129 mouse substrains and the ES cells derived from them recently has been documented (24). In contrast to the Pax-2 mouse produced in ref. 20, which does not show loss of midbrain and cerebellar tissue, the Pax-21neu mutant (12) seems to lose the cerebellum and the inferior colliculi. Because all of these mutations appear to be null alleles of the Pax-2 gene this discrepancy is unclear at present. In this study we show that in C57BL/6, the most commonly used mouse strain for genetic analysis because of its relatively low degree of phenotypic variability, a possible Pax-2 phenotype in the brainstem is rescued by its family member Pax-5 expressed with an overlapping pattern. However, on deletion of both genes we get an invariable phenotype, suggesting that both genes cooperate in midbrain/hindbrain development and can activate the same target genes.
Neither the Pax-2 Nor the Pax-5 Mutation Show Gross Midbrain/Hindbrain Patterning Defects.
Both the Pax-2 and the Pax-5 single mutants look normal as revealed by in situ hybridization for marker gene expression (Figs. 1 and 2) and morphological observations (not shown). The normal development of the brainstem in the Pax-2 and the subtle alterations described for the Pax-5 single mutants (3) suggest that there is normal development of the midbrain/hindbrain organizing center in the absence of either Pax-2 or Pax-5. Two key findings confirm the morphological inspection. First, the territory of expression of Otx-2 (23) showed a sharp boundary at the isthmus (in the mutants as in the wild-type animals), indicating that the positioning of the boundary was not affected (Fig. 2). Second, no Wnt-1 positive cells could be found caudal to the Otx-2 expressing cells, suggesting that there was no perturbation in boundary formation in the single mutants (25) (Fig. 1). The same results have been obtained in embryos at e10.5 (data not shown). Our results indicate that the hypothesized cascade of induction responsible for the regionalization of mesencephalon and metencephalon (10) is not altered in the absence of Pax-2 even if this is the earliest known transcription factor specifically expressed in that region (11). However, we cannot rule out effects in marker gene expression in the time window between the onset of Pax-2 and Pax-5 expression. Possible effects at that early stages are, however, suppressed after the onset of Pax-5, leading to completely normal development in 70% of the animals (this study).
Gene Dosage Effects in Brain Regionalization.
Our results indicate that there is a dosage of Pax genes crucial for the normal development of the midbrain/metencephalon region. One single functional allele of Pax-2 is necessary and sufficient for normal regionalization of the brain in the absence of Pax-5. The fact that one single allele of Pax-5 is not sufficient to regulate brainstem development in the absence of Pax-2 is most likely because of the earlier expression of Pax-2 in this region.
These results do not seem to be completely in agreement with the results previously obtained with the Krd mutant mice (15). Krd is a transgene induced mutation of the genome, resulting in a 7-cM deletion of chromosome 19 comprising, among other genes, the whole Pax-2 locus (16). Mice carrying compound Krd +/− Pax-5 −/− mutations show a complete loss of the posterior midbrain and cerebellum, which is similar to the En-1 mutant mice (15). This loss has been interpreted to mean that at least one Pax-5 allel is required for normal development of the midbrain and metencephalon. The discrepancy observed to the results reported here could be because of the different genetic backgrounds used. Alternatively, because the Krd deletion is largely uncharacterized, it could be that besides lacking Pax-2, the mutants also lack one or more genes necessary for the development of the brain. This alternative interpretation would be in agreement with the results of the present study, which reveals that Pax-2, the earliest expressed Pax gene in the region of the presumptive isthmus (11), is the limiting factor and the major regulator in the cascade of events leading to the development of the midbrain and cerebellum.
Cooperation of Pax-2 and Pax-5 in Brain Regionalization.
The normal brain phenotype that we have obtained at high frequency with the Pax-2 mutant, together with the reported data on the Pax-5 mutant brain, suggest that both proteins could have a similar biological function and share in part the same targets. This functional substitution, which had been suggested already on the basis of the analysis of the compound Krd/Pax-5 mutation (17) is definitely proven by the fact that, in sharp contrast to the single Pax-2 and Pax-5 mutants, the compound Pax-2/Pax-5 mutant shows an alteration of the tectum and a deletion of the cerebellum. These structures are derived from the regions of early expression of these genes in the neural plate primordium. A similar type of cooperation between two transcription factors, En-1 and En-2, in the development of the midbrain and cerebellum, also has been demonstrated (14).
One has to notice, however, that terms like “cooperation” and “rescue” are misleading out of the context of distinct areas within the nervous system development; the Pax-2 single mutant mice exhibit complete bilateral coloboma, do not have kidneys, and die at birth (9, 20), even if the midbrain and cerebellum seem to develop normally.
Acknowledgments
We are indebted to Dr. Gail Martin for the Fgf-8 probe, Dr. Antonio Simeone for the Otx-2 probe, and Dr. Francesco Cecconi for critical reading of the manuscript. This work was supported by the Max-Planck Society. P.U. was on leave of absence from the Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Prague.
ABBREVIATION
- en
embryonic day
References
- 1.Puelles L. Brain Behav Evol. 1995;46:319–337. doi: 10.1159/000113282. [DOI] [PubMed] [Google Scholar]
- 2.Alvarado-Mallart R M. J Neurobiol. 1993;24:1341–1355. doi: 10.1002/neu.480241007. [DOI] [PubMed] [Google Scholar]
- 3.Urbánek P, Wang Z-Q, Fetka I, Wagner E F, Busslinger M. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 4.Thomas K R, Capecchi M R. Nature (London) 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 5.Crossley P H, Martínez S, Martin G R. Nature (London) 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 6.McMahon A P, Bradley A. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 7.McMahon A P, Joyner A L, Bradley A, McMahon J A. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- 8.Joyner A, Herrup K, Auerbach B A, Davis C A, Rossant J. Science. 1991;251:1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- 9.Torres M, Gomez-Pardo E, Gruss P. Development (Cambridge, UK) 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- 10.Joyner A L. Trends Genet. 1996;12:15–20. doi: 10.1016/0168-9525(96)81383-7. [DOI] [PubMed] [Google Scholar]
- 11.Rowitch D H, McMahon A P. Mech Dev. 1995;52:3–8. doi: 10.1016/0925-4773(95)00380-j. [DOI] [PubMed] [Google Scholar]
- 12.Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Spörle R, Schughart K. Proc Natl Acad Sci USA. 1996;93:1–6. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurst W, Auerbach A B, Joyner A L. Development (Cambridge, UK) 1994;120:2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- 14.Hanks M, Wurst W, Anson-Cartwright L, Auerbach A B, Joyner A L. Science. 1995;269:679–684. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- 15.Urbánek P, Fetka I, Meisler M H, Busslinger M. Proc Natl Acad Sci USA. 1997;94:5703–5708. doi: 10.1073/pnas.94.11.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller S A, Jones J M, Boyle A, Barrow L L, Killen P D, Green D G, Kapousta N V, Hitchcock P F, Swank R T, Meisler M H. Genomics. 1994;23:309–320. doi: 10.1006/geno.1994.1506. [DOI] [PubMed] [Google Scholar]
- 17.Stoykova A, Gruss P. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson D G. In: Essential Developmental Biology: A Practical Approach. Stern C D, Holland P W H, editors. Oxford: IRL; 1993. pp. 257–274. [Google Scholar]
- 19.Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nature (London) 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- 20.Torres M, Gomez-Pardo E, Dressler G R, Gruss P. Development (Cambridge, UK) 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 21.Ang S-L. Nature (London) 1996;380:25–27. doi: 10.1038/380025a0. [DOI] [PubMed] [Google Scholar]
- 22.Bally-Cuif L, Alvarado-Mallart R-M, Darnell D K, Wassef M. Development (Cambridge, UK) 1992;115:999–1009. doi: 10.1242/dev.115.4.999. [DOI] [PubMed] [Google Scholar]
- 23.Millet S, Bloch-Gallego E, Simeone A, Alvarado-Mallart R M. Development (Cambridge, UK) 1996;122:3785–3797. doi: 10.1242/dev.122.12.3785. [DOI] [PubMed] [Google Scholar]
- 24.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 25.Bally-Cuif L, Wassef M. Development (Cambridge, UK) 1994;120:3379–3394. doi: 10.1242/dev.120.12.3379. [DOI] [PubMed] [Google Scholar]