Abstract
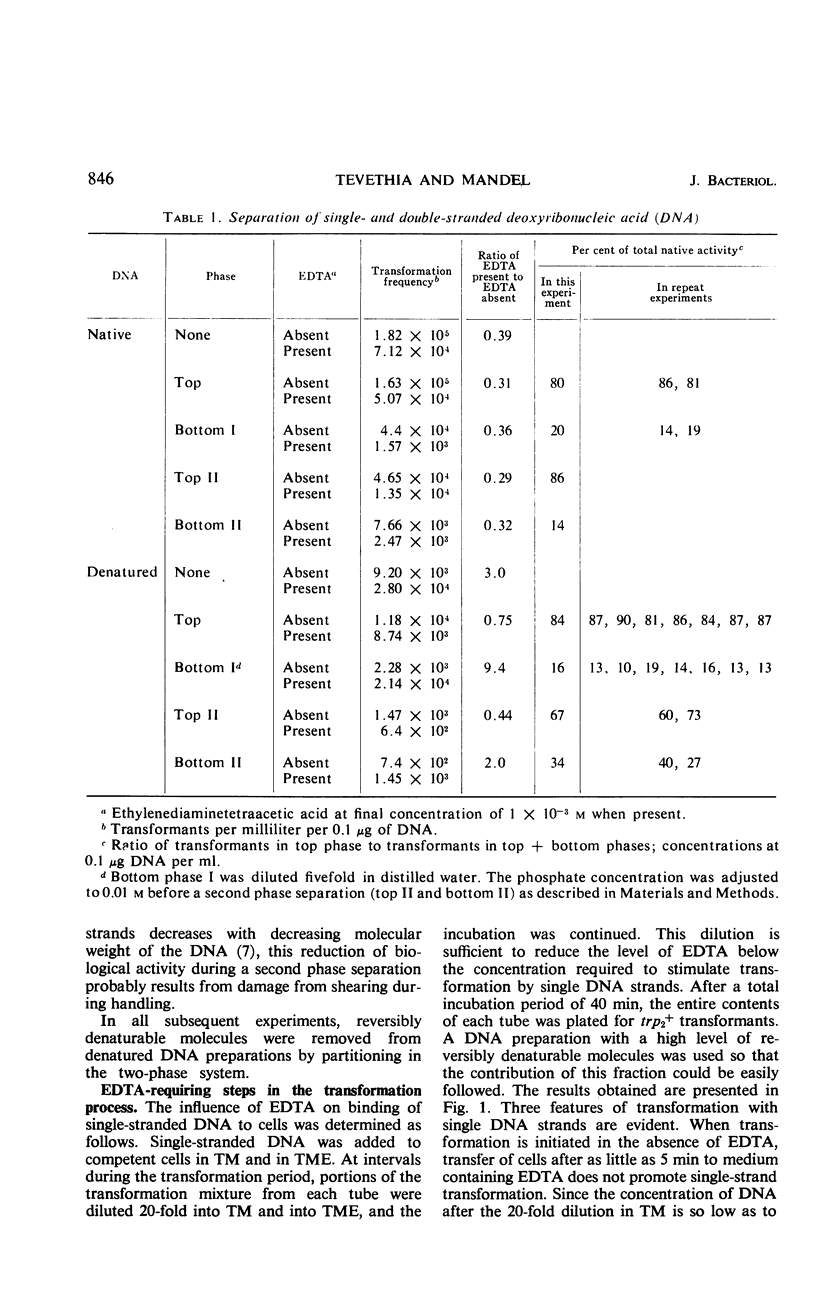
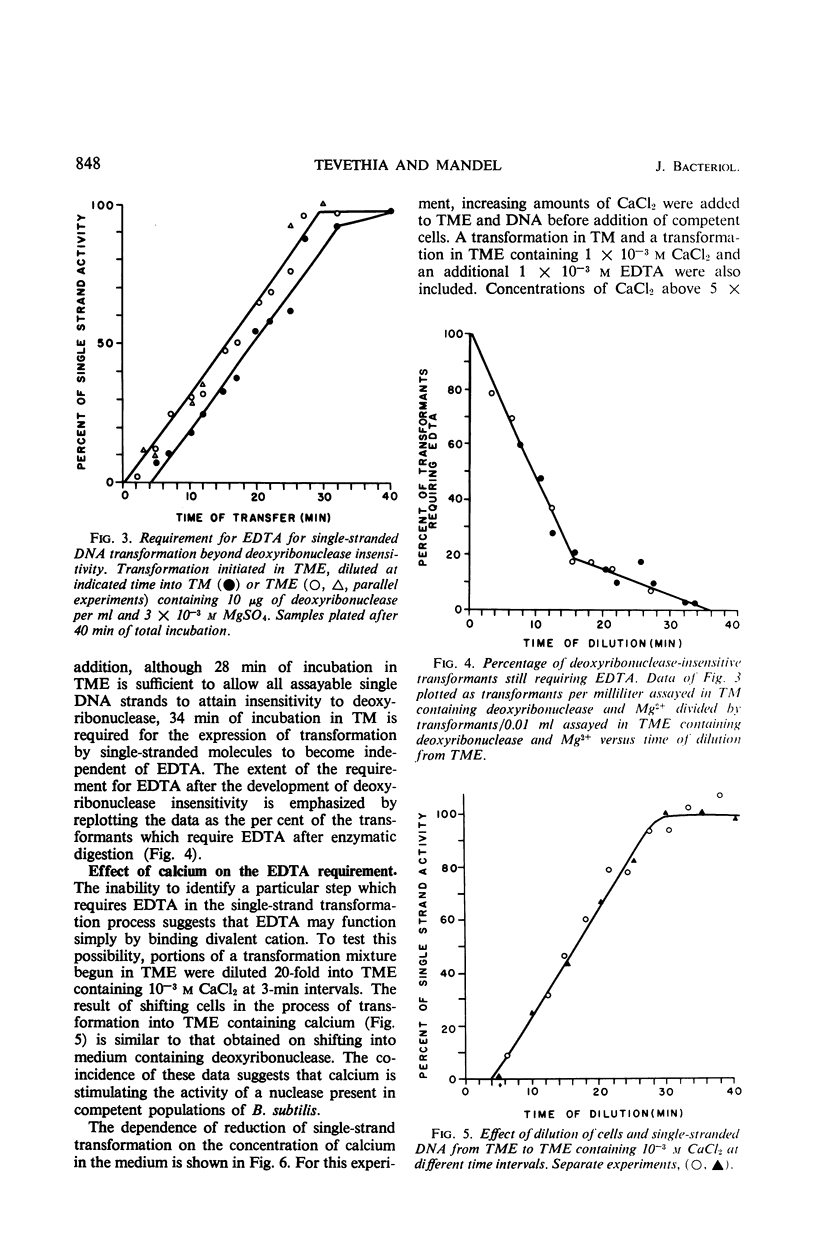
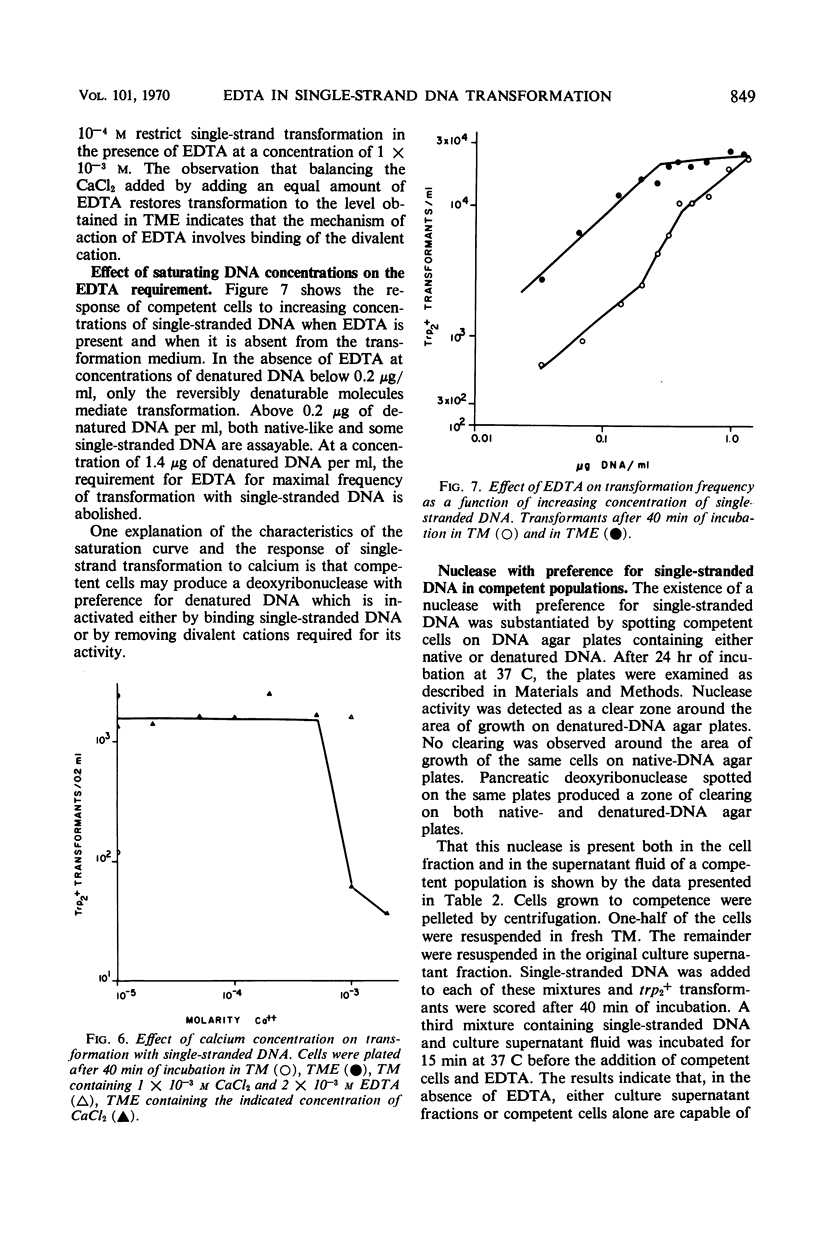
The ethylenediaminetetraacetate (EDTA) requirement for transformation of Bacillus subtilis with single-stranded deoxyribonucleic acid (DNA) was examined. The results indicate that a chelating agent such as EDTA is a stringent requirement for transformation with single DNA strands only at nonsaturating DNA concentrations, and that EDTA, when required, must be present during several steps in the transformation process and appears to insure the survival of single-stranded DNA by rendering a nuclease in competent populations inactive.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Doty P. Characterization of a naturally occurring, cross-linked fraction of DNA. 1. Nature of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):379–403. doi: 10.1016/0022-2836(68)90017-x. [DOI] [PubMed] [Google Scholar]
- Alberts B. M. Efficient separation of single-stranded and double-stranded deoxyribonucleic acid in a dextran-polyethylene glycol two-phase system. Biochemistry. 1967 Aug;6(8):2527–2532. doi: 10.1021/bi00860a033. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. Cellular site in Bacillus subtilis of a nuclease which preferentially degrades single-stranded nucleic acids. J Bacteriol. 1966 Mar;91(3):1004–1011. doi: 10.1128/jb.91.3.1004-1011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Ravin A. W. Mechanism of the deoxyribonucleic acid helping effect during transformation. J Mol Biol. 1968 May 14;33(3):873–891. doi: 10.1016/0022-2836(68)90325-2. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bernardi G. Residual transforming activity of denatured Haemophilus influenzae DNA. J Mol Biol. 1968 Mar 14;32(2):437–451. doi: 10.1016/0022-2836(68)90020-x. [DOI] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Doty P. Residual activity of denatured transforming DNA of Haemophilus influenzae: a natrually occurring cross-linked DNA. J Mol Biol. 1968 Mar 14;32(2):423–435. doi: 10.1016/0022-2836(68)90019-3. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Goodgal S. H. Further studies on transformation with single-stranded DNA of Hemophilus influenzae. J Mol Biol. 1967 Sep 14;28(2):247–259. doi: 10.1016/s0022-2836(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Goodgal S. H. Uptake of "single-stranded" DNA in Hemophilus influenzae and its ability to transform. J Mol Biol. 1966 Apr;16(2):317–327. doi: 10.1016/s0022-2836(66)80175-4. [DOI] [PubMed] [Google Scholar]
- Rownd R., Green D. M., Sternglanz R., Doty P. Origin of the residual transforming activity of denatured Bacillus subtilis DNA. J Mol Biol. 1968 Mar 14;32(2):369–377. doi: 10.1016/0022-2836(68)90016-8. [DOI] [PubMed] [Google Scholar]
- Rownd R., Green D. M., Sternglanz R., Doty P. Origin of the residual transforming activity of denatured Bacillus subtilis DNA. J Mol Biol. 1968 Mar 14;32(2):369–377. doi: 10.1016/0022-2836(68)90016-8. [DOI] [PubMed] [Google Scholar]


